|
Case Report
Percutaneous irreversible electroporation in combination with radiofrequency ablation in the treatment of non-resectable hepatocellular carcinoma: A case report
1 Department of Cardiac Surgery and Transplantation, Section of Clinical Ecointervention, Monaldi Hospital, AORN Ospedali dei Colli, Piazzale Ettore Ruggieri snc, Naples, Italy
2 Department of Critical Care Area, Unit of Anesthesia and Intensive Care, Monaldi Hospital, AORN Ospedali dei Colli, Piazzale Ettore Ruggieri snc, Naples, Italy
Address correspondence to:
Enrico Ragone
MD, Department of Cardiac Surgery and Transplantation, Chief of Section of Clinical Ecointervention, Monaldi Hospital, AORN Ospedali dei Colli, Piazzale Ettore Ruggieri snc, 80131, Naples,
Italy
Message to Corresponding Author
Article ID: 100055Z06SS2018
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Scarica S, Sanges F, Giuseppe R, Ragone E. Percutaneous irreversible electroporation in combination with radiofrequency ablation in the treatment of non-resectable hepatocellular carcinoma: A case report. Case Rep Int 2018;7:100055Z06SS2018.ABSTRACT
Introduction: There are more option of treatments of hepatocellular carcinoma (HCC), that is currently the sixth most common cancer worldwide. As only 10–20% of patients are candidates for surgery (resection or transplantation), ablative therapies such as Radiofrequency (RF) and Microwave (MW) ablation are both widely used techniques, but tumors adjacent to heat-sensitive structures such as the gallbladder, major bile ducts, portal vein, and hepatic veins are difficult to destroy. Irreversible electroporation (IRE) is a new non-thermal ablation technique for pancreatic and liver tumors that generates electrical fields leading to cell death through apoptosis. In this way IRE prevents collateral damage to other tissues, such as blood vessels, bile ducts, nerves, gallbladder and other vital organs which often occur in thermal ablation techniques.
Case Report: We report the treatment of an inoperable HCC with a maximum diameter of 40 mm in left lobe at the 4th hepatic segment, adjacent to the gallbladder in a 75-year-old female with hepatitis C–related cirrhosis. It was used for the first time a dual thermal and nonthermal ablative technique: ultrasound–guided irreversible electroporation ablation (IRE) following ultrasound–guided radiofrequency ablation (RFA) in the same session. We got complete response at 1, 3 and 6 months follow-up without evidenced of local tumor progression or metastatic disease.
Conclusion: Combination therapy plays an important role in the treatment of unresectable HCC, because near vital structures, but additional research is necessary to assess further the safety, efficacy and long-term outcomes for this combination therapy.
Keywords: Hepatocellular carcinoma, Irreversible electroporation, Non-resectable hepatocellular carcinoma, Radiofrequency ablation
INTRODUCTION
Hepatocellular carcinoma (HCC) is currently the sixth most common cancer worldwide and the third leading cause of cancer-related death, numbering over 700,000 new cases annually [1],[2],[3].
Hepatic resection (HR) and liver transplantation (LT) are the main curative option. But at the time of initial diagnosis, only 10–20% of patients are candidates for surgery mainly because of the multiplicity of the lesions that often occurs in a background of chronic liver disease, bad liver function, deteriorating general condition and by the location of the tumor. Consequently, several curative treatment options have been developed over the years just as effective as image-guided ablation techniques. Ablative therapies such as Radiofrequency (RF) and Microwave (MW) ablation are both widely used techniques for primary and secondary liver cancer. Both techniques kill target lesions by gathering thermal energy, but the generated heat is difficult to control due to thermal fluctuation from blood circulation which affects the local heat (“heat sink effect”) [4],[5]. This is particularly evident for tumors adjacent to heat-sensitive structures such as the gallbladder, major bile ducts, portal vein and hepatic veins.
Recently, irreversible electroporation (IRE) has been introduced as a novel technique for image-guided tumor ablation. IRE is non-thermal ablation technique for pancreatic and liver tumors that generates electrical fields capable of altering the cell membrane’s electrical potential and disrupting the lipidic bilayer structure, thereby creating small nanopores through which micromolecules or macromolecules can enter or exit the cell and interfering with cell homeostasis and leading to cell death through apoptosis [6]. The non-thermal electrical characteristic of IRE prevents collateral damage to other tissues, such as blood vessels, bile ducts, nerves, gallbladder, and other vital organs which often occur in thermal ablation techniques [6]. Thus, for HCC adjacent to large vessels, IRE ablation may be a more appropriate choice, especially for those tumors smaller than 3 cm, because larger tumors are generally associated with greater biological activity and aggressiveness and they represents an independent risk factor for local recurrence [7].
In this case we report the treatment of an inoperable hepatocarcinoma with a maximum diameter of 40 mm treated for the first time with dual thermal and non-thermal ablative technique: ultrasound–guided irreversible electroporation ablation (IRE) following ultrasound–guided radiofrequency ablation (RF) in the same session.
CASE REPORT
A 75-year-old female was admitted to our institution in November 2017 for hepatocellular carcinoma (HCC). She also had hepatitis C–related cirrhosis, chronic bronchopneumopathy and mild mitral insufficiency.
The patient was without other complaints at the time of presentation, denying nausea, vomiting, change in bowel habits, jaundice, decreased appetite and early satiety.
At the time of treatment, laboratory testing revealed the following levels: serum albumin, 3,1 g/dl; serum total bilirubin, 1,6 mg/dl; prothrombine activity rate 94%; platelets 83.000 μ/l; white blood cells 3800 μ/l, AST 146 U/L, ALT 138 U/L, ALP 262 U/L, GGT 130 U/L, serum creatinine 0,6 mg/dl, urea 26 mg/dl, blood sodium 143 mEq/L; α-feto-protein 30,8 ng/ml. The patient’s hepatic function was given a Child-Pugh score of A6 points and a Model for End-Stage Liver Disease score of 9.
Ultrasound liver demonstrating hypoechoic mass in the 4th hepatic segment (Figure 1). Preoperative contrast-enhanced ultrasonography (CEUS) revealed a nodule hyperenhanced in arterial phase and washed out in portal phase with a maximum diameter of 40 mm in left lobe at the 4th hepatic segment, adjacent to the gallbladder, and therefore it was unresectable. Computed tomography (CT) confirmed the diagnosis. There was no evidence of extrahepatic metastasis. The pathological examination demonstrated well- to moderately differentiated HCC.
In the same session local treatment using IRE+ RFA was planned in agreement with the patient. Prior to the procedure, the patient received general anesthesia. Induction is performed with propofol (about 3 mg/kg), remifentanyl (0.10-0.25 g/kg/m) and the curarization is guaranteed with rocuronium (0.6 mg/ kg). The maintenance of anaesthesia is obtained with desfluorane (MAC 0,6) and remifentanyl (0.05-0.25 g/kg/m). We used the standard monitoring (ECG, NIBP, plethysmography). Furthermore, monitoring of curarization is obtained with TOFcuff Monitor® (RGB medical, Madrid, Spain) while depth of anaesthesia is monitored trough BIS monitor® (Covidien-Medtronic, Minneapolis, USA).
All registered parameters were in the normal range before starting ablation. For this reason, deep curarization is required for all the duration of procedure and monitoring is fundamental as well as specific rocuronium antagonism obtained through sugammadex at the end of the procedure.
After general anesthesia, we inserted two 15-cm electrodes in the most cranial portion of the tumor with real-time ultrasound guidance (MyLab40; Esaote Biomedica, Genova, Italy), using a 3.5-MHz sector probe (CA431, 1-8 MHz). The two electrodes (NanoKnife System; AngioDynamics, Latham, New York, USA), had a 2-cm exposed tip and they were placed parallel within the tumor 1.5–2.0 cm apart, and 90 pulses of 2,500 V/cm3 were applied between the electrode pairs.
Following RFA using the Cool-tip® RF System (Mygen M 3004, RF Medical Co., Ltd, Seoul, Korea) was performed under real-time ultrasonographic guidance (MyLab40; Esaote Biomedica, Genova, Italy), using a 3.5-MHz sector probe (CA431, 1-8 MHz). A guide device incorporated into the ultrasound probe was used for RF electrode placement.
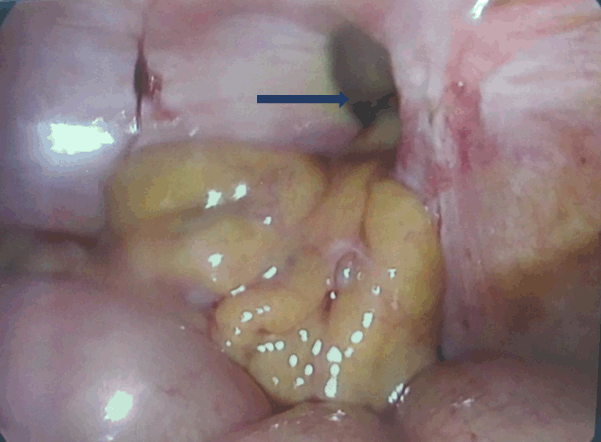
We inserted two RF electrodes (a 15-cm long, 17-gauge internally cooled with 2,5 cm of exposed metallic tip) in the most caudal portion of the tumor was applied the RF current (Mygen M 3004, RF Medical Co., Ltd, Seoul, Korea) for a duration of seven minutes and respecting the margin from the hepatic capsule and from the gallbladder (Figure 2). Complete ablation of the tumor mass together was achieved with a 1-cm margin as showed by CEUS post-RF. No problems were encountered during the entire procedure the duration of which was 1h.
According to the departmental protocol, systemic quinolone (levofloxacin 500 mg QD ev) antibiotic therapy and painkillers (paracetamol 1 g BID ev) were administered after the treatment and for the following 2–3 days.
The day after procedure, venous sampling showed a moderate increase in transaminases (AST from 146 to 315 U/L, ALT from 38 to 170 U/L) with slight signs for cholestasis (total bilirubin 2,10 mg/dl). These values normalized after 15 days.
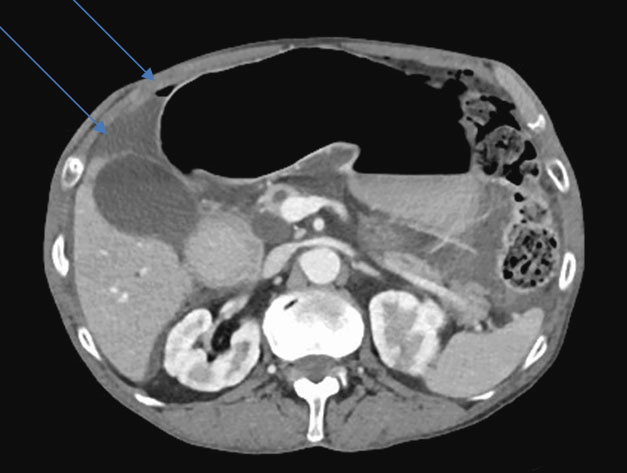
The ultrasonography post-RF didn‘t show fluid surrounding the treated lesion or in abdomen. One day post-RF, the patient underwent CEUS of the liver which showed a large region of induced necrosis encompassing the vicinity of the original lesion. The patient was discharged home three day after the procedure and returned for a follow-up examination one month later. Computed tomography (CT) scan was performed four weeks and six months after the procedure: it confirmed complete tumor ablation (Figure 3, Figure 4).
DISCUSSION
During the past two decades, radiofrequency ablation (RFA), a thermal ablative modality, has been the mainstay of treatment offered to patients who are not suitable for resection or do not meet criteria for transplantation. Paralysis and cardiac synchronization are necessary to avoid muscle contractions and arrhythmias, respectively [8]. Various studies have proven that RFA is a safe therapeutic option with both low mortality and morbidity and has been widely accepted as effective means of minimally invasive treatment for liver tumors [9],[10].
But this thermal ablation has several limitations such as the risk of thermal damage to the tissue of adjacent structures or the blood vessels [11] or incomplete ablation of tumors adjacent to “heat sink” with negative influence on the long-term outcome [12]. Furthermore, cholecystitis or gallbladder perforation with bile leak can occour as a serious potential complication if liver tumour is adjacent to it [13],[14].
Therefore, until a few years ago HCC localized near blood vessels or bile ducts or gallbladder were inoperable both surgically and ablation techniques. Irreversible electroporation (IRE) is a newly developed non-thermal ablative technique that uses high-current electrical pulses to induce pore formation of the cell lipid bilayer, leading to cell death.
IRE processes its own advantages especially including avoiding the heat sink and sparing vital structures adjacent to tumor, such as blood vessels, gallbladder and bile ducts. IRE seems to be an attractive alternative option for tumors near gallbladder, the porta hepatis or other main vessels in which thermal ablations are risky to be performed [15].
There is no other case in the literature that use both techniques (IRE + RFA) in the same session. In our case IRE was chosen to minimize any potential of thermal injury, but IRE ablation efficacy is promising for tumors smaller than 3 cm. But in our case HCC was 40 mm of diameter, for this reason we used IRE in the most cranial portion of the tumor, adjacent to the gallbladder, to preserve it, and RFA in the most caudal portion of the tumor far from gallbladder. This non-thermal ablative technique is promising, safety and increasingly used in the clinic, and it is a new choice for liver cancer in recent years [16],[17],[18].
This case demonstrates that when IRE is utilized in combination to RF ablation, it is efficacy ablative option to treat inoperable tumors adjacent to the gallbladder and with a maximum diameter >3 cm. We got complete response at 1, 3, 6 and 12 months follow-up without evidenced of local tumor progression or metastatic disease.
CONCLUSION
The safety of IRE for ablation of hepatocellular carcinoma (HCC) has been established, but this case report is the first that utilizes a combination therapy (IRE+RF) in the same session. In this case vital structures and blood vessels were all well-preserved although they were really close to the ablated area. The combination therapy plays an important role in the treatment of unresectable HCC, because near vital structures, but additional research is necessary to assess further the safety, efficacy, and longterm outcomes for this combination therapy.
REFERENCE
1.
Balogh J, Victor D 3rd, Asham EH, et al. Hepatocellular carcinoma: A review. J Hepatocell Carcinoma 2016 Oct 5;3:41–53. [CrossRef]
[Pubmed]

2.
Bruix J, Sherman M; American association for the study of liver diseases. Management of hepatocellular carcinoma: An update. Hepatology 2011 Mar;53(3):1020–2. [CrossRef]
[Pubmed]

3.
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010 Dec 15;127(12):2893–917. [CrossRef]
[Pubmed]

4.
Joosten J, Ruers T. Local radiofrequency ablation techniques for liver metastases of colorectal cancer. Crit Rev Oncol Hematol 2007 May;62(2):153–63. [CrossRef]
[Pubmed]

5.
Yoon J, Cho J, Kim N, et al. High-frequency microwave ablation method for enhanced cancer treatment with minimized collateral damage. Int J Cancer 2011 Oct 15;129(8):1970–8. [CrossRef]
[Pubmed]

6.
Lee EW, Chen C, Prieto VE, Dry SM, Loh CT, Kee ST. Advanced hepatic ablation technique for creating complete cell death: Irreversible electroporation. Radiology 2010 May;255(2):426–33. [CrossRef]
[Pubmed]

7.
Niessen C, Igl J, Pregler B, et al. Factors associated with short-term local recurrence of liver cancer after percutaneous ablation using irreversible electroporation: A prospective single-center study. J Vasc Interv Radiol 2015 May;26(5):694–702. [CrossRef]
[Pubmed]

8.
Nielsen K, Scheffer HJ, Vieveen JM, et al. Anaesthetic management during open and percutaneous irreversible electroporation. Br J Anaesth 2014 Dec;113(6):985–92. [CrossRef]
[Pubmed]

9.
Van Amerongen MJ, van der Stok EP, Fütterer JJ, et al. Short term and long term results of patients with colorectal liver metastases undergoing surgery with or without radiofrequency ablation. Eur J Surg Oncol 2016 Apr;42(4):523–30. [CrossRef]
[Pubmed]

10.
Fonseca AZ, Saad WA, Ribeiro MA Jr. Complications after radiofrequency ablation of 233 hepatic tumors. Oncology 2015;89(6):332–6. [CrossRef]
[Pubmed]

11.
Zheng RN, You ZJ, Lin SH, et al. Efficacy of percutaneous radiofrequency ablation for the treatment of hepatocellular carcinoma. Genet Mol Res 2015 Dec 22;14(4):17982–94. [CrossRef]
[Pubmed]

12.
Ng KK, Poon RT, Lo CM, Yuen J, Tso WK, Fan ST. Analysis of recurrence pattern and its influence on survival outcome after radiofrequency ablation of hepatocellular carcinoma. J Gastrointest Surg 2008 Jan;12(1):183–91. [CrossRef]
[Pubmed]

13.
Kim SW, Rhim H, Park M, et al. Percutaneous radiofrequency ablation of hepatocellular carcinomas adjacent to the gallbladder with internally cooled electrodes: Assessment of safety and therapeutic efficacy. Korean J Radiol 2009 Jul–Aug;10(4):366–76. [CrossRef]
[Pubmed]

14.
Lee J, Rhim H, Jeon YH, et al. Radiofrequency ablation of liver adjacent to body of gallbladder: Histopathologic changes of gallbladder wall in a pig model. AJR Am J Roentgenol 2008 Feb;190(2):418–25. [CrossRef]
[Pubmed]

15.
Cannon R, Ellis S, Hayes D, Narayanan G, Martin RC 2nd. Safety and early efficacy of irreversible electroporation for hepatic tumors in proximity to vital structures. J Surg Oncol 2013 Apr;107(5):544–9. [CrossRef]
[Pubmed]

16.
Dollinger M, Müller-Wille R, Zeman F, et al. Irreversible electroporation of malignant hepatic tumors - Alterations in venous structures at subacute follow-up and evolution at mid-term follow-up. PLoS One 2015 Aug 13;10(8):e0135773. [CrossRef]
[Pubmed]

17.
Dollinger M, Zeman F, Niessen C, et al. Bile duct injury after irreversible electroporation of hepatic malignancies: Evaluation of MR imaging findings and laboratory values. J Vasc Interv Radiol 2016 Jan;27(1):96–103. [CrossRef]
[Pubmed]

18.
Cheung W, Kavnoudias H, Roberts S, Szkandera B, Kemp W, Thomson KR. Irreversible electroporation for unresectable hepatocellular carcinoma: Initial experience and review of safety and outcomes. Technol Cancer Res Treat 2013 Jun;12(3):233–41. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Sabrina Scarica - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Federica Sanges - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Giuseppe Rossi - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Enrico Ragone - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this case report.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2018 Sabrina Scarica et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.