|
Case Report
Myoepithelial carcinoma of the elbow diagnosed by immunohistochemistry: Case report of an uncommon neoplasm with metastatic recurrence
1 Department of Pathology, American University of the Caribbean, USA
Address correspondence to:
Madhura Mahapatra
Department of Pathology, American University of the Caribbean,
USA
Message to Corresponding Author
Article ID: 100062Z06MM2019
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Mahapatra M, Lambert T, El-Mallah AR, Balbi A, Aziz M. Myoepithelial carcinoma of the elbow diagnosed by immunohistochemistry: Case report of an uncommon neoplasm with metastatic recurrence. Case Rep Int 2019;8:100062Z06MM2019.ABSTRACT
Myoepithelial carcinomas have a nature of occurring infrequently. While the histopathological characterization is limited and so few cases have been reported, it is important to keep myoepithelial carcinoma on the list of differential diagnoses and should be ruled out like any other soft tissue tumor.
Keywords: Immunohistochemistry, Metastasis, Myoepithelial, Recurrence
INTRODUCTION
Myoepithelial carcinoma occurring in soft tissue is quite uncommon but is more well-known as an occurrence in the salivary gland [1]. The heterogeneous nature of cell type and architecture composing a soft tissue myoepithelial carcinoma (STMC) is as such due to variance in cytology, mitotic figures, and invasive growth. The prominent features suggestive of an STMC include tumor cells that may be epithelioid, spindle, clear, or plasmacyoid, with numerous mitoses [2]. Other main features are the presence of moderate to severe atypia with vesicular nuclei and prominent nucleoli. This alone is not sufficient for accurate diagnosis and emphasizes the importance of immunohistochemical staining [3]. Here we present an uncommon case of an infrequent malignancy, due to its location and make-up.
CASE REPORT
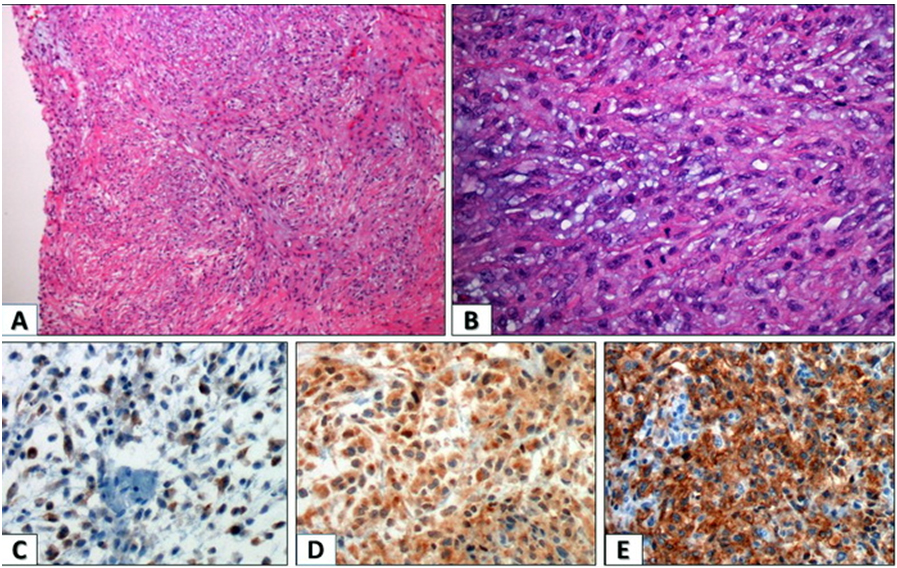
A 44-year-old female patient presented with palpable firm left elbow mass. The mass had been noted seven months prior and the size of the mass had recently increased to 6.0×5.0 cm. There was no association with pain and the patient sought medical attention due to enlarging size and discomfort. On examination, the oval firm mass was fixed on the underlying ulna without tenderness. The mass was heterogeneous echoic solid mass on sonography. Laboratory blood values were within normal levels. A local excision was performed. The mass was located in the subcutaneous soft tissue, focally fixed to the ulnar bone and measured 6.0×5.0×3.0 cm. The mass was lobulated and the cut surface was white gray tan with multiple necrotic foci. The tumor was confined to the soft tissue without dermal invasion. On microscopic examination, the tumor presented with various histologic growth patterns including solid sheets, trabecular, reticular patterns, and short fascicle with myxoid and hyalinized stroma. Furthermore, histological study displayed a malignant infiltrating neoplasm arising from dermal tissue into the subcutaneous tissue and extending into the underlying bone. The elbow mass was multilobulated, composed of chords or nests of epithelioid, ovoid, plasmacytoid, or spindle cells with variable reticular architecture and chondromyxoid stroma weaved with collagenous or hyalinized stroma. When assessing the tumor for malignancy, it appeared to have high-grade malignancy with low-grade areas displaying transition into high-grade. Additionally, prominent cellular atypia and cellular pleomorphism were noted, along with mitosis exceeding 10 mitosis/HPF. Immunohistochemistry studies were essential to determine the definitive diagnosis and to rule out all other possible entities (Figure 1 A, Figure 1B). Immunohistochemical staining was done on the excised tissue sections and was strongly positive for Vimentin, CD138, and GFAP, and was focally positive for epithelial membrane antigen (EMA). Staining was negative for AE1/AE3, HMW CK 906, Caldesmon, Calponin, CD31, CD56, CD57, Desmin, Myogenin, S100 SMA, Cytokeratin CK-903, p63, and synaptophysin (Figure 1 C, Figure 1D, Figure 1E). Based on the patient’s age, sex, gross presentation, cytology, and immunohistochemical staining, the diagnosis is established as a malignant myoepithelial soft tissue tumor of the elbow (myoepithelial carcinoma). While cytopathological study and immunohistochemical staining are not completely diagnostic of soft tissue tumors as a myoepithelial carcinoma, these render other diagnoses less likely. For follow-up on the patient, surgical excision of the tumor was performed, but it was noted that surgical margins were multifocally involved by the tumor—this is suggestive of invasion of normal tissue border, thereby suggesting presence of residual malignancy. Patient had postsurgical radiation therapy, but seven months thereafter, reoccurrence occurred at the same site. Another surgical excision was performed but there was no involvement of the surgical border noted, and the patient was started on chemotherapy and remained disease-free for four years. After this period, metastasis to the lungs was discovered, hence the patient began two cycles of chemotherapy but was then lost to follow-up. Based on the statistics of recurrence and metastasis in Hornick’s study, our patient’s case study mirrors the recurrence and metastasis patterns.
DISCUSSION
Soft tissue myoepithelial carcinoma (STMC) is a rare salivary gland tumor composed entirely of myoepithelial cells that exhibit a dual epithelial and smooth muscle phenotype. Epithelial–myoepithelial carcinoma is a rare malignant tumor that typically arises in a salivary gland and consists of both an epithelial and myoepithelial component. They are predominantly found in the parotid gland and represent approximately 1% of salivary gland tumors.
According to Hornick et al. (2003), of the 101 STMC cases that were studied, most tumors were lobulated and had variable reticular architecture with chondromyxoid or hyalinized stroma; the mid-grade and high-grade tumors (due to increased cellular atypia) presented with epithelioid or spindle cells with coarse chromatin, prominent and large nucleoli, or nuclear pleomorphism [4]. While cytopathological patterns indicate a range of cellular atypia and nuclear disruption, it is insufficient to diagnose STMC, hence the addition of immunohistochemistry staining is required. Epithelial staining was reactive for all cases which had viable tissue to stain: 93% stained positive for keratin (AE1/AE3 or PAN-K); 87% positive for S-100 protein; 86% positive for calponin; 63% positive for epithelial membrane antigen (EMA); 46% positive for glial fibrillary acidic protein (GFAP); 36% positive for smooth muscle actin; 23% positive for p63; and 14% positive for desmin. Of the malignant cases of myoepithelial carcinoma, 42% recurred locally and 32% metastasized, while four patients died of metastatic tumor [4]. Even with the combination of cytopathological studies and immunohistochemistry staining, there is difficulty in defining universally accepted criteria for malignant myoepithelial carcinomas as compared to their salivary gland counterparts. Invasion beyond the tumor capsule, cellular atypia, and mitotic rate have been reported as useful markers to assess the malignancy of such tumors [4].
Based on Hornick’s study of 101 cases of myoepithelial tumors, 3 of 101 cases studied occurred in the elbow (3%), making this an uncommon site for myoepithelial neoplasms to occur [4]. Among 31 cytologically malignant cases, 13 recurred locally (42%) and 10 metastasized (32%). There is no general consensus on a standardized treatment for STMC due to its rare and aggressive nature. A combination therapy that is aggressive may be key in treatment of this particular tumor as incorrect diagnosis and ignorance about a potentially aggressive disease such as this one can lead to undertreatment of patients. Part of successful diagnosis or early intervention remains in the swift use of cytopathological studies along with immunohistochemical stains.
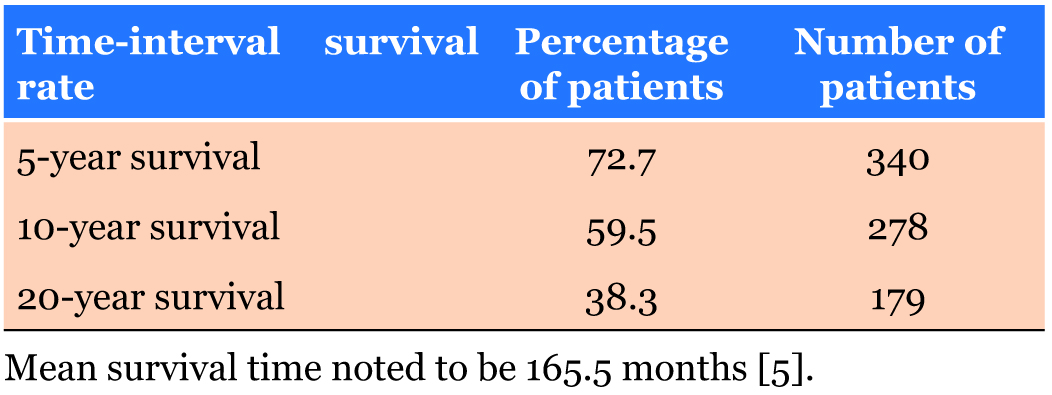
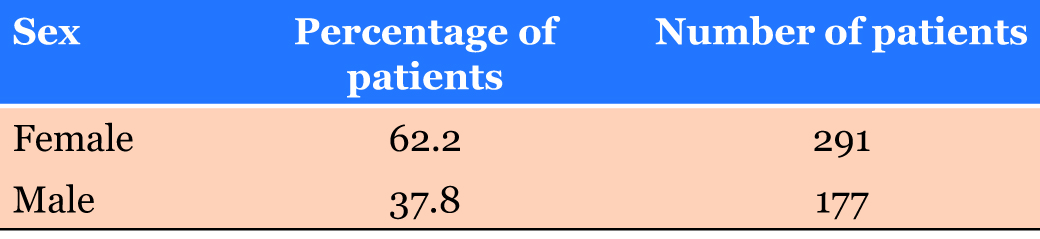
A large study by Mitchell Gore analyzing 468 cases of epithelial–myoepithelial carcinoma between 1973–2014 provided valuable population-based survival analysis [5]. These data included mean survival rate of epithelial– myoepithelial carcinoma (Table 1), most common sites of occurance of epithelial–myoepithelial carcinoma (Table 2), epithlial–myoepithelial carcinoma occurrence by sex (Table 3), and epithelial–myoepithelial occurrence by race (Table 4).
CONCLUSION
We bring this case forward to shed light on the importance of including STMC in the list of differential diagnoses for soft tissue tumors, as well as being conscious of cellular atypia, mitotic activity, and tumor infiltration suggesting the malignant potential of myoepithelial carcinomas. We must also keep in mind that while STMC is rare, it behaves synonymously to its more well-known salivary gland counterpart.
REFERENCE
1.
Choi CH, Chu YC, Kim L, et al. Myoepithelial carcinoma of soft tissue: A case report and review of the literature. Korean J Pathol 2014;48(6):413–7. [CrossRef]
[Pubmed]

2.
Acikalin MF, Pasaoglu O, Cakli H, Gürbüz K, Canaz F. Malignant myoepithelioma of the palate: A case report with review of the clinicopathological characteristics. Yonsei Med J 2009;50(6):848–51. [CrossRef]
[Pubmed]

3.
4.
Hornick JL, Fletcher CD. Myoepithelial tumors of soft tissue: A clinicopathologic and immunohistochemical study of 101 cases with evaluation of prognostic parameters. Am J Surg Pathol 2003;27(9):1183–96.
[Pubmed]

5.
Gore MR. Epithelial-myoepithelial carcinoma: A population-based survival analysis. BMC Ear Nose Throat Disord 2018;18:15. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Madhura Mahapatra - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Travis Lambert - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Abdal-Rahman El-Mallah - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Andressa Balbi - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Mohamad Aziz - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2019 Madhura Mahapatra et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.