|
Case Report
Pulmonary embolism identified prior to anesthesia induction in a low-risk patient
1 MD, Clinical Assistant Professor, Anesthesiology and Pain Medicine, Wonju Severance Christian Hospital, Wonju, Gangwon-do, Republic of Korea
2 MD, Resident, Anesthesiology and Pain Medicine, Wonju Severance Christian Hospital, Wonju, Gangwon-do, Republic of Korea
3 MD, PhD, Professor, Anesthesiology and Pain Medicine, Yonsei University Wonju College of Medicine, Wonju, Gangwon-do, Republic of Korea
Address correspondence to:
Hyun Kyo Lim
Department of Anesthesiology and Pain Medicine, Yonsei University Wonju College of Medicine, 20 Ilsan-ro, Wonju, Gangwon-do 26426,
Republic of Korea
Message to Corresponding Author
Article ID: 100079Z06JP2020
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Park J, Lee EB, Hong D, Lee KH, Lim HK. Pulmonary embolism identified prior to anesthesia induction in a low-risk patient. Case Rep Int 2020;9:100079Z06JP2020.ABSTRACT
Introduction: Early detection and treatment of pulmonary embolism (PE) are critical for reducing morbidity and mortality of patients. However, diagnosis can be difficult if the symptoms are not clear.
Case Report: A 21-year-old male patient visited the emergency room with multiple fractures, including femur fractures. Three days later, the planned operation was scheduled. On the day of surgery, the operation was canceled due to clinical diagnosis of PE because oxygen saturation on the pulse oximeter was 71–76% without dyspnea. Later, he was diagnosed with PE of the right lower lobe based on computed tomography (CT) pulmonary angiography, and surgery was performed without complications after low-molecular weight heparin treatment.
Conclusion: Even if a patient is low risk and does not have symptoms suspicious of PE, careful preoperative patient monitoring and preoperative anesthetic evaluation are important for identifying additional patient risks.
Keywords: Fracture, Oxygen saturation, Pulmonary embolism, Pulse oximeter
INTRODUCTION
Pulmonary embolism (PE) is the presence of mechanical obstruction in the pulmonary artery and its branches due to substances, such as thrombus, tumor, air, and fat. It is associated with various clinical symptoms based on degree and progression. The most common symptoms are dyspnea and chest pain. Other symptoms that may occur include cough, hemoptysis, orthopnea, arrhythmia in rare cases, presyncope, syncope, and hemodynamic collapse. However, clinical diagnosis may be difficult because symptoms may not appear as expected [1],[2]. We report a case of PE in the presence of multiple fractures including that of the femur. The patient was clinically diagnosed based on low oxygen saturation by pulse oximetry after arriving at the operating room and confirmed subsequent examinations.
CASE REPORT
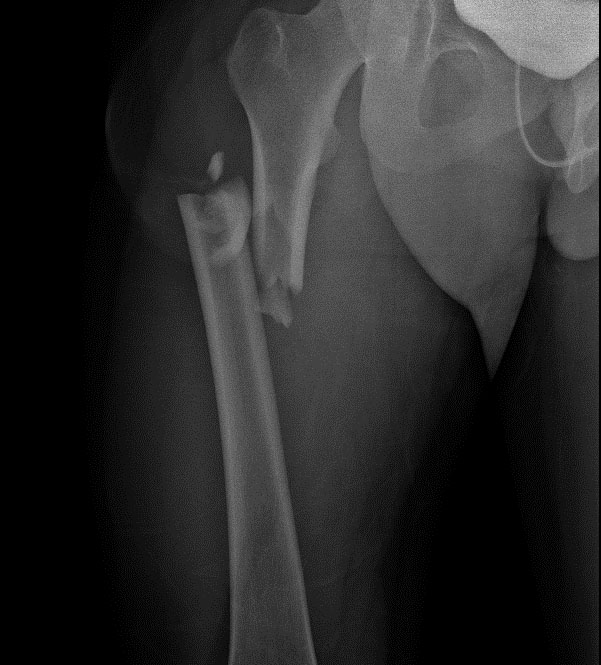
A 21-year-old male patient was admitted after a car accident two days prior to admission. At the time of the visit, X-ray showed right femur fracture (Figure 1), third and fourth metacarpal bone fractures of the left hand, and scaphoid fracture of the right hand. The patient did not have notable past medical history.
The O2 saturation was measured at 99% by pulse oximetry in the emergency room at the time of the visit. Ground glass opacity was seen in the right lung on chest CT, and the diagnosis was pneumonia. Other than fever up to 38.6 ℃ at hospitalization and on the day of surgery, there were no other specific abnormalities. However, there were no upper airway symptoms, such as cough, sputum, rhinorrhea, or dyspnea. On the day of surgery, the patient complained of momentary pain in his left anterior chest wall.
On the second day of admission, a fracture reduction operation was scheduled. Premedication was not performed. Upon arrival in the operating room, blood pressure was 160–180/70–80 mmHg, heart rate was 70–100/min, and body temperature was 37.0 °C. The pulse oximetry sensor was placed on the left second finger to measure oxygen saturation and showed SpO2 between 71% and 76%. The device was changed to the disposable attaching type probe from the reusable glove sensor to eliminate potential device measurement errors, but the result was the same. The measurement site was changed to the left foot, and the same result was obtained. SpO2 increased to 98–100% at 6 L/min oxygen supply through the facial mask but decreased to 73% when the oxygen supply was stopped. Arterial blood gas analysis (ABGA) was performed at the left femoral artery in room air and showed severe hypoxemia with 74% saturation and 37 mmHg of PaO2. No rash or petechiae were observed in the patient’s body. Since PE such as deep vein thrombosis, fat embolism, and venous air embolism could not be ruled out, surgery was postponed and discussed after evaluation.
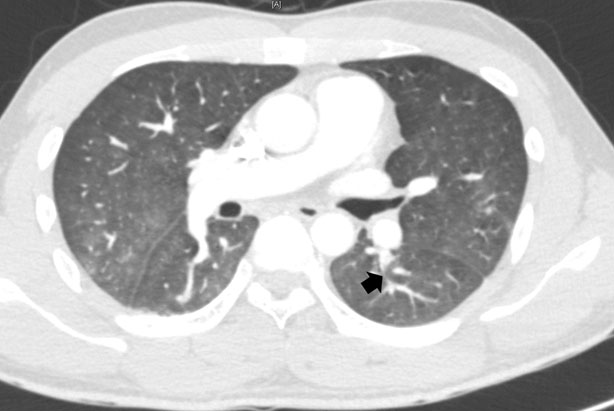
The patient was transferred to a traumatic intensive care unit (TICU) with 5 L/min oxygen supply. On the Trauma Intensive Care Unit (TICU), ABGA showed PaO2 77.8 mmHg, saturation 95.9%, alveolar-arterial (A-a) gradient 190.6 mmHg, and D-dimer 1100 ng/mL. Emergency CT pulmonary angiography showed focal contrast filling defect at the superior segmental artery of the left lower lobe (LLL) (Figure 2). A pulmonologist and thoracic surgeon were consulted based on diagnostic impression of acute pulmonary thromboembolism. Emergency CT lower extremity venography was performed but showed no evidence of deep vein thrombosis.
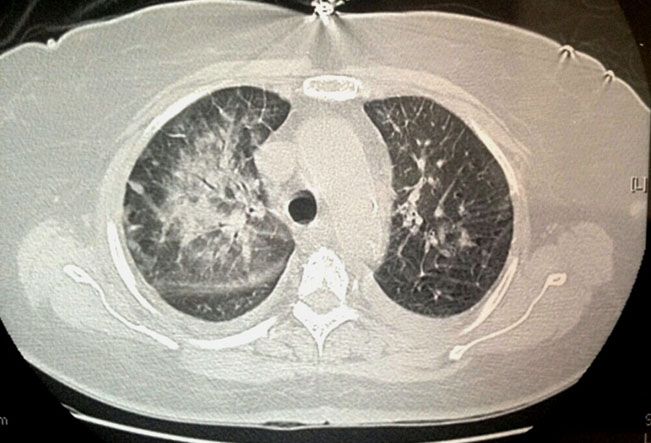
Enoxaparin was administered subcutaneously for seven days after TICU admission for PE treatment. The patient subsequently showed stable oxygen saturation with PaO2 143.2 mmHg in FiO2 0.36 and PaO2 156.1 mmHg in FiO2 0.32 in the TICU. On the 10th day of TICU admission, the operation proceeded under general anesthesia without any problems. On the fourth postoperative day, ABGA was performed in room air and showed PaO2 106.0 mmHg, which is in the normal range. Chest CT on the fifth postoperative day showed resolution of the previous focal contrast filling defect at the superior segmental artery of the LLL (Figure 3). The patient was discharged on the 13th postoperative day without complication.
DISCUSSION
Pulmonary embolism is more common in elderly patients with underlying diseases [3],[4],[5]. According to Naess et al. [6], the incidence rate of PE was three times higher in patients over 70 years than for patients aged between 45 and 69 years, and the rate for patients 45–69 years was three times higher than in patients aged 20–44 years. The incidence rate was 0.13 in the 20–24-year-old patient group.
Representative risk factors for pulmonary embolism include corticosteroids, diabetes mellitus, disseminated intravascular coagulation, hypothyroidism, myocardial disease, neoplasm, trauma, sepsis, and indwelling venous catheters [7]. In this case, the patient did not have any underlying medical disease, and the only risk factor for PE was immobility for three days due to traumatic fractures. According to Menaker et al. [8], pulmonary embolism occurred in 94 of 35,424 trauma patients, and lower leg and femur trauma, as in this case, accounted for 38% of those events. In addition, about 65% of embolisms occur within two weeks of immobilization; as in this case, the frequency of occurrence within two days or less is about 7% [9].
The patient complained only of chest pain without dyspnea or cough. Although pleuritic chest pain is reported in approximately 66% of PE, the prior trauma provided the possibility of a differential diagnosis, such as contusion [9].
Vital signs that can indicate a diagnosis include hypoxia, which is a typical and important clinical feature of PE. However, not all cases of hypoxia have a PE diagnosis, and normal arterial oxygen tension is seen in massive PE in some cases [10]. Typical blood gas changes associated with PE include hypoxemia, hypocapnia, and increased A-a gradient. Using an A-a gradient can be more sensitive than judging PE by hypoxemia alone and people with an A-a gradient >53 mmHg have a poor prognosis [11],[12],[13],[14]. Because O2 saturation was 99% by pulse oximetry and there was no dyspnea when the patient visited the emergency room, further O2 saturation measurement and ABGA were not performed during general ward admission.
On the day of scheduled surgery, the initial O2 saturation measured in the operating room ranged from 71% to 76%. When the patient was given a facial mask and supplied 6 L/min oxygen, the saturation rose to 98–100% but decreased again in the room air, which suggested hypoxia and hypoxemia. The ABGA showed 74% saturation, severe hypoxemia of PaO2 37 mmHg, and an increased A-a gradient 69.0 mmHg. The O2 saturation measurement and ABGA were not performed during general ward admission; therefore, we could not determine when the hypoxia worsened, and this delayed detection of the PE event.
Wells criteria can be used when PE is suspected in hemodynamically stable patients. The calculated scores can be used to predict the probability of PE: values above 6 points indicate high risk (78.4%), values between 2 and 6 points indicate moderate risk (27.8%), and fewer than 2 points indicate low risk (3.4%). In the modified Wells criteria, a score below 4 points indicates that PE is unlikely, and a score above 4 points indicates that PE is likely. If the score is “low risk” or “moderate risk” and D-dimer concentration is less than 500 ng/mL, PE can be excluded. If the concentration of D-dimer exceeds 500 ng/mL or the Wells criteria score is “high risk,” CT pulmonary angiography should be performed immediately [9],[15]. According to the Wells criteria, the patient’s heart rate is over 100 times and the score is 1.5, indicating low risk. It also corresponds to “unlikely pulmonary embolism” based on the modified Wells criteria.
In this case, although the Wells criteria indicated a low probability, acute PE was suspected based on history of trauma and immobility. The blood test after postponing surgery showed A-a gradient of 190.6 mmHg and D-dimer of 1100 ng/mL; emergency CT pulmonary angiography focal contrast filling defect at the superior segmental artery of the LLL. Based on these results, a diagnosis of acute PE was concluded, with appropriate treatment, the operation was performed safely after oxygen saturation was stabilized.
The limitation in this case is that the potential for fat embolism has not been evaluated. The diagnosis of fat embolism is made by clinical diagnosis. Laboratory tests are used only to help in clinical diagnosis [16]. The clinical diagnosis of fat embolism requires typical clinical symptoms and exclusion of other possible diagnoses. The typical clinical symptoms of fat embolism are petechial rash, decreased level of consciousness, and shortness of breath [17]. The patient complained only of dyspnea and showed no petechial rash or decreased level of consciousness. Moreover, dyspnea is also a major symptom of PE. Based upon the immediately performed CT pulmonary angiography which showed focal contrast filling defect at the superior segmental artery of the left lower lobe (LLL), we diagnosed the patient as PE. We did not proceed with additional laboratory tests, such as urine analysis, because the case did not meet the clinical diagnosis of fat embolism. It would have been better if laboratory tests and consultations on fat embolism were conducted for a clear differential diagnosis.
CONCLUSION
Low-risk patients with mild symptoms, such as in this case, cannot be excluded from the possibility of acute complications, such as PE. Preoperative patient monitoring and preoperative anesthetic evaluation should be performed with care to identify and treat any potential risks for the patient.
REFERENCE
1.
Doherty S. Pulmonary embolism: An update. Aust Fam Physician 2017;46(11):816–20.
[Pubmed]

2.
3.
Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: A 25-year population-based study. Arch Intern Med 1998;158(6):585–93. [CrossRef]
[Pubmed]

4.
Tagalakis V, Patenaude V, Kahn SR, Suissa S. Incidence of and mortality from venous thromboembolism in a real-world population: The Q-VTE Study Cohort. Am J Med 2013;126(9):832.e13–21. [CrossRef]
[Pubmed]

5.
Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States,1979–1998: An analysis using multiple-cause mortality data. Arch Intern Med 2003;163(14):1711–7. [CrossRef]
[Pubmed]

6.
Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrøm J. Incidence and mortality of venous thrombosis: A population-based study. J Thromb Haemost 2007;5(4):692–9. [CrossRef]
[Pubmed]

7.
Goggs R, Benigni L, Fuentes VL, Chan DL. Pulmonary thromboembolism. J Vet Emerg Crit Care (San Antonio) 2009;19(1):30–52. [CrossRef]
[Pubmed]

8.
Menaker J, Stein DM, Scalea TM. Incidence of early pulmonary embolism after injury. J Trauma 2007;63(3):620–4. [CrossRef]
[Pubmed]

9.
Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991;100(3):598–603. [CrossRef]
[Pubmed]

10.
Huet Y, Lemaire F, Brun-Buisson C, et al. Hypoxemia in acute pulmonary embolism. Chest 1985;88(6):829–36. [CrossRef]
[Pubmed]

11.
Riedel M. Diagnosing pulmonary embolism. Postgrad Med J 2004;80(944):309–19. [CrossRef]
[Pubmed]

12.
Dalen JE. Pulmonary embolism: What have we learned since Virchow? Natural history, pathophysiology, and diagnosis. Chest 2002;122(4):1440–56. [CrossRef]
[Pubmed]

13.
Hsu JT, Chu CM, Chang ST, et al. Prognostic value of arterial/alveolar oxygen tension ratio (a/APO2) in acute pulmonary embolism. Circ J 2007;71(10):1560–6. [CrossRef]
[Pubmed]

14.
Hsu JT, Chu CM, Chang ST, et al. Prognostic role of alveolar-arterial oxygen pressure difference in acute pulmonary embolism. Circ J 2006;70(12):1611–6. [CrossRef]
[Pubmed]

15.
van Belle A, Büller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 2006;295(2):172–9. [CrossRef]
[Pubmed]

16.
Taviloglu K, Yanar H. Fat embolism syndrome. Surg Today 2007;37(1):5–8. [CrossRef]
[Pubmed]

17.
Akhtar S. Fat embolism. Anesthesiol Clin 2009;27(3):533–50. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Jihyoung Park - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Eun Bi Lee - Conception of the work, Design of the work, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Donguei Hong - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Kwang Ho Lee - Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Hyun Kyo Lim - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2020 Jihyoung Park et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.