|
Clinical Image
PPI-induced hypomagnesemia with secondary hypoparathyroidism and posterior reversible encephalopathy syndrome restricted to the cerebellum
1 Emeritus Professor of Medicine, Lehigh Valley Health Network, Morsani School of Medicine, University of South Florida, Allentown, PA, USA
Address correspondence to:
Yehia Y Mishriki
MD, Emeritus Professor of Medicine, Department of Medicine, Morsani School of Medicine, University of South Florida, Lehigh Valley Health Network–One City Center, 707 Hamilton Street, Allentown, PA 18101,
USA
Message to Corresponding Author
Article ID: 100085Z06YM2020
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Mishriki YY. PPI-induced hypomagnesemia with secondary hypoparathyroidism and posterior reversible encephalopathy syndrome restricted to the cerebellum. Case Rep Int 2020;9:100085Z06YM2020.ABSTRACT
No Abstract
Keywords: Hypomagnesemia, Posterior reversible encephalopathy syndrome (or PRES), Proton pump inhibitor (or PPI), Secondary hypoparathyroidism
CASE REPORT
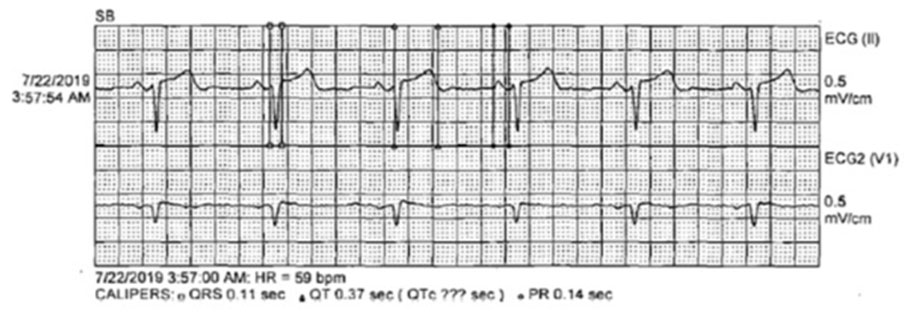
A 42-year-old male presented with anorexia and dyspepsia. His physical exam was unremarkable. His medication list was notable for the chronic use of a proton pump inhibitor (PPI). A chemistry panel revealed a calcium of 7.5 mg/dL. The next day he presented with a severe headache and dizziness. Laboratory studies revealed a calcium of 6.5 mg/dL, magnesium 0.4 mg/dL, and phosphorus of 3.8 mg/dL. Fractional urinary excretion of magnesium was 0.2%. A parathyroid hormone (PTH) level was 22 pg/mL (nl: 14–72 pg/mL). Magnetic resonance imaging (MRI) of the brain (Figure 1) revealed moderate vasogenic edema within the left cerebellar hemisphere and mild vasogenic edema within the right cerebellar hemisphere with a mass effect on the inferior aspect of the fourth ventricle.
The PPI was discontinued. The magnesium and calcium levels normalized with magnesium replacement and the patient’s symptoms quickly resolved. A repeat MRI showed significant improvement in the cerebellar edema.
DISCUSSION
There have been several reports of hypomagnesemia associated with acute/subacute cerebellitis or cerebellar edema [1],[2],[3],[4],[5],[6],[7],[8]. Nearly all had resolution of clinical signs and symptoms, as well as of MRI changes, with magnesium replacement. There have also been reports of posterior reversible encephalopathy syndrome (PRES) in the setting of PPI-induced hypomagnesemia. Ross Russell et al. reported on three patients with cerebellar syndromes associated with hypomagnesemia with typical PRES findings on MRI who had rapid resolution of both symptoms and MRI changes with magnesium replacement. Two of the three patients had been on a PPI and the third was an alcoholic [7]. Rouco Axpe et al. reported a case of a 43-year-old male with probable PPI-induced hypomagnesemia and vasogenic edema limited to the cerebellum [8]. Li et al. described two patients with PRES limited to the cerebellum and identified nine other cases in the literature [9]. Of note, a retrospective study identified low magnesium levels in many patients during the acute phase of PRES, regardless of its etiology [10],[11].
Magnesium homeostasis is maintained by a balance between intestinal absorption and renal excretion. Intestinal absorption occurs via a paracellular passive transport as well as an active transcellular mechanism involving transient receptor potential melastatin 6 and 7 (TRPM6/7) channels. Magnesium deficiency increases the expression of TRPM6/7 in the intestine which enhances active magnesium transport [12]. It has been shown that PPIs decrease intestinal luminal pH which alters theTRPM6/7 channel affinity for magnesium resulting in decreased absorption. In patients with PPI-induced hypomagnesemia, urinary excretion of magnesium is low implicating gastrointestinal impairment of magnesium absorption [13]. Danziger et al. confirmed the association of PPIs with hypomagnesemia in hospitalized patients [14]. The inappropriately normal level of parathyroid hormone in our patient supports the diagnosis of secondary hypoparathyroidism. It is known that hypomagnesemia both impairs release of PTH and decreases peripheral tissue sensitivity to PTH leading to a functional secondary hypoparathyroidism [15].
The mechanism whereby magnesium deficiency causes PRES is not known but magnesium deficiency has been shown to promote inflammation. Studies in rat models and humans have linked inflammatory illnesses with hypomagnesemia. Normally, magnesium activates the thiamine pyrophosphate (TTP)-dependent riboswitch which results in increased synthesis of thiazole. Products of thiazole metabolism have been shown to have anti-inflammatory and neuroprotective effects and low concentrations of thiazole can, therefore, promote a pro-inflammatory state. Hypomagnesemia has also be shown to result in elevated levels of tumor necrosis factor alpha (TNFα) which leads to the transcription of interleukin-6 and interferon-c, two cytokines with pro-inflammatory actions [16]. Furthermore, magnesium plays a role in the suppression of N-methyl-D-aspartate (NMDA) receptors and its deficiency leads to NMDA receptor hyperexcitability. This is further amplified by activation of γ-aminobutyric acid (GABA) receptors which are, in part, regulated by magnesium. The increased release of glutamate by NMDA-receptor rich neurons, possibly by the inhibition of voltage-gated calcium channels, leads to an influx of calcium ions into neurons triggering an increased production of toxic reactive oxygen radicals [12]. Magnesium is also intricately linked to vascular function. Endothelial cells contribute to the inflammation and endothelial dysfunction associated with hypomagnesemia in part due to the impairment of release of nitric oxide from those cells [17].
CONCLUSION
In summary, PPIs are effective but generally over utilized medications in the treatment of chronic gastroesophageal reflux. It has recently become more appreciated that PPI use is linked to a variety of complications, some of which are quite serious. Hypomagnesemia is a well-documented but underappreciated complication of chronic PPI use. The described patient documents a case of PPI-induced prolonged hypomagnesemia leading to PRES limited to the cerebellar hemispheres as well as a secondary hypoparathyroid state.
REFERENCE
1.
Flanagan EP, Rabinstein AA, Kumar N, Schroeder K, Kantarci OH. Fulminant cerebellitis with radiological recurrence in an adult patient with Crohn’s disease. J Neurol Sci 2014;336(1–2):247–50. [CrossRef]
[Pubmed]

2.
Santos AF, Sousa F, Rodrigues M, Ferreira C, Soares-Fernandes J, Maré R. Reversible cerebellar syndrome induced by hypomagnesemia. Neurology and Clinical Neuroscience 2015;3(5):190–1. [CrossRef]

3.
Boulos MI, Shoamanesh A, Aviv RI, Gladstone DJ, Swartz RH. Severe hypomagnesemia associated with reversible subacute ataxia and cerebellar hyperintensities on MRI. Neurologist 2012;18(4):223–5. [CrossRef]
[Pubmed]

4.
Te Riele MGE, Verrips A. Severe hypomagnesaemia causing reversible cerebellopathy. Cerebellum 2014;13(5):659–62. [CrossRef]
[Pubmed]

5.
de Mendonca JLF, Barbosa H, Viana SL, Freitas FMO, Viana MACB, Ferreira ACL. Pseudotumoural hemicerebellitis: Imaging findings in two cases. Br J Radiol 2005;78(935):1042–6. [CrossRef]
[Pubmed]

6.
Sedehizadeh S, Keogh M, Wills AJ. Reversible hypomagnesaemia-induced subacute cerebellar syndrome. Biol Trace Elem Res 2011;142(2):127–9. [CrossRef]
[Pubmed]

7.
Ross Russell AL, Prevett M, Cook P, Barker CS, Pinto AA. Reversible cerebellar oedema secondary to profound hypomagnesaemia. Pract Neurol 2018;18(4):311–4. [CrossRef]
[Pubmed]

8.
Rouco Axpe I, Almeida Velasco J, Barreiro Garcia JG, Urbizu Gallardo JM, Mateos Goñi B. Hypomagnesemia: A treatable cause of ataxia with cerebellar edema. Cerebellum 2017;16(5–6):988–90. [CrossRef]
[Pubmed]

9.
Li D, Lian L, Zhu S. Isolated cerebellar involvement in posterior reversible encephalopathy syndrome. J Neurol Sci 2015;357(1–2):101–5. [CrossRef]
[Pubmed]

10.
Chardain A, Mesnage V, Alamowitch S, et al. Posterior reversible encephalopathy syndrome (PRES) and hypomagnesemia: A frequent association? Rev Neurol (Paris) 2016;172(6–7):384–8. [CrossRef]
[Pubmed]

11.
Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, et al. Proton pump inhibitors linked to hypomagnesemia: A systematic review and meta-analysis of observational studies. Ren Fail 2015;37(7):1237–41. [CrossRef]
[Pubmed]

12.
de Baaij JHF, Hoenderop JGJ, Bindels RJM. Magnesium in man: Implications for health and disease. Physiol Rev 2015;95(1):1–46. [CrossRef]
[Pubmed]

13.
William JH, Danziger J. Proton-pump inhibitor-induced hypomagnesemia: Current research and proposed mechanisms. World J Nephrol 2016;5(2):152–7. [CrossRef]
[Pubmed]

14.
Danziger J, William JH, Scott DJ, et al. Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int 2013;83(4):692–9. [CrossRef]
[Pubmed]

15.
Clarke BL, Brown EM, Collins MT, et al. Epidemiology and diagnosis of hypoparathyroidism. J Clin Endocrinol Metab 2016;101(6):2284–99. [CrossRef]
[Pubmed]

16.
Chandrasekaran NC, Weir C, Alfraji S, Grice J, Roberts MS, Barnard RT. Effects of magnesium deficiency – More than skin deep. Exp Biol Med (Maywood) 2014;239(10):1280–91. [CrossRef]
[Pubmed]

17.
Shechter M. Magnesium and cardiovascular system. Magnes Res 2010;23(2):60–72. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Yehia Y Mishriki - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthor declares no conflict of interest.
Copyright© 2020 Yehia Y Mishriki. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.