|
Case Report
Nebulized tranexamic acid directly causing transient left bundle branch block
1 DO, Internal Medicine Resident, Mercy Medical Center-North Iowa, 1000 4th St SW, Mason City, IA 50401, USA
2 MBBS, Medical Research Assistant, Mercy Medical Center-North Iowa, 1000 4th St SW, Mason City, IA 50401, USA
3 MD, Department of Cardiology-Electrophysiology, Mercy Medical Center-North Iowa, 1000 4th St SW, Mason City, IA 50401, USA
4 MD, Department of Critical Care, Mercy Medical Center-North Iowa, 1000 4th St SW, Mason City, IA 50401, USA
Address correspondence to:
Katharine Lasley Woods
Mercy-ONE North Iowa, 1000 4th St SW, Mason City, IA 50401,
USA
Message to Corresponding Author
Article ID: 100086Z06KW2020
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Woods KL, Nagraj S, Germano N, Koranne K, Summerfield DT. Nebulized tranexamic acid directly causing transient left bundle branch block. Case Rep Int 2020;9:100086Z06KW2020.ABSTRACT
Introduction: Tranexamic acid (TXA) is an antifibrinolytic with applications in preventing potentially life-threatening hemorrhage.
Case Report: A 39-year-old male presented with massive hemoptysis and, during treatment with TXA, developed a transient left bundle branch block (LBBB). He was recently initiated on warfarin, but then developed a pulmonary hemorrhage. Along with standard treatments, it was decided to administer inhaled TXA to assist with hemodynamic stabilization. On persistent telemetry monitoring in the critical care unit, during administration of his first nebulized dose of TXA, a new onset LBBB was observed. Electrocardiogram (EKG) findings resolved to normal sinus rhythm within 30 minutes of TXA discontinuation. A second round of inhaled TXA was administered 8 hours later during which the LBBB reoccurred. It resolved within approximately 30 minutes without demonstration of troponin increase.
Conclusion: To our knowledge, this is the first described instance of EKG changes seen after administration of inhaled TXA; therefore, caution should be exercised particularly in those with concomitant cardiovascular disease.
Keywords: Bundle branch block, Hemoptysis, Tranexamic acid
INTRODUCTION
Nebulized tranexamic acid (TXA) as a treatment modality for controlling massive (>200 mL/24 hour) and non-massive hemoptysis is relatively novel despite the widely recognized utility of its oral and intravenous counterparts [1],[2]. Oral TXA adverse effects, according to data from clinical trials and post-marketing surveillance, include seizures, headaches, backache, abdominal pain, nausea, vomiting, diarrhea, fatigue, pulmonary embolism, deep vein thrombosis, anaphylaxis, impaired color vision, and other visual disturbances [3]. However, literature delineating side effects and therapeutic efficacy of inhaled TXA is sparse. We consider this discussion particularly useful to suggest the potential cardiac side effects of nebulized TXA. Although localized drug forms can have high therapeutic efficacy, as in this case where hemoptysis was arrested after two doses of nebulized TXA, mindful use of this formulation is recommended as a cardiac conduction event, such as a left bundle branch block (LBBB), can have significant clinical consequences.
CASE REPORT
A 39-year-old male presented to the critical care unit for airway monitoring with moderate hemoptysis (50–200 mL/24 hour). His past medical history was significant for lupus anticoagulant pulmonary embolism (PE), deep venous thrombosis (DVT), and multifocal ischemic stroke, for which he was bridged with enoxaparin until warfarin was therapeutic. He was recently admitted ten days prior for rapidly progressive mediastinal lymphadenopathy associated with pulmonary Sporobolomyces. Two days prior to readmission, his previously prescribed rivaroxaban was discontinued due to a drug interaction with itraconazole and he was started on warfarin and enoxaparin.
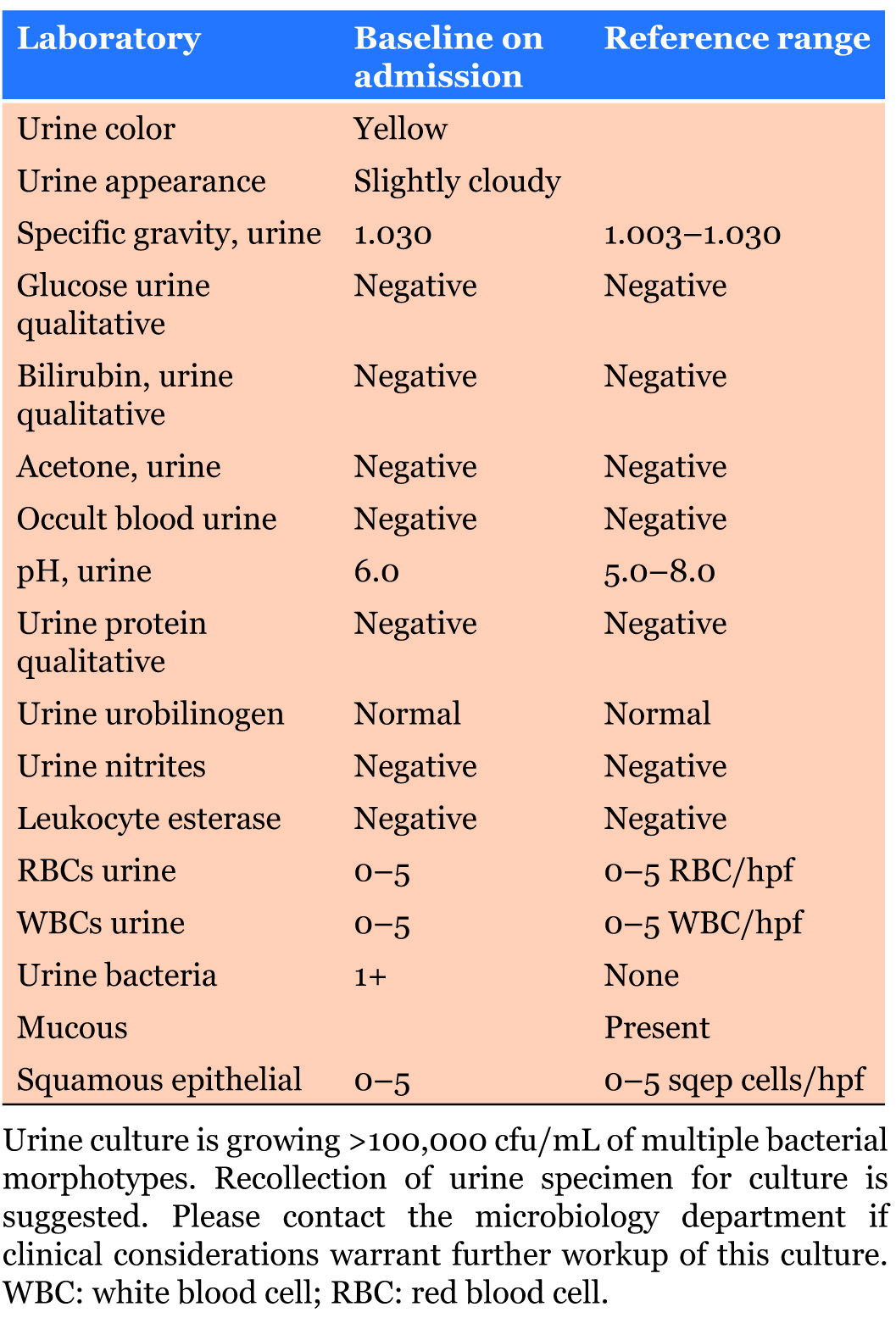
On the day of admission the patient developed massive hemoptysis and was transferred to the medical intensive care unit. The patient remained hemodynamically stable throughout his hospital stay. Initial hemoglobin was 14.8 g/dL (normal range 11.7–16.1 g/dL) with no significant drop (>2 mg/dL) during hemoglobin checks every 6 hours. Taking into consideration the patient’s multiple comorbidities, he was a high risk for systemic thromboembolism, positive for lupus anticoagulant coagulopathy in the setting of a recent embolic stroke, recurrent deep venous thrombosis, and pulmonary embolism. Thus, it was determined that locally directed TXA as opposed to its systemic form was indicated. During administration of his first nebulized dose of TXA, telemetry demonstrated a LBBB, confirmed by an EKG. Serial high sensitivity troponins trended downward 95.5 ng/mL (normal range 0–13 ng/mL) at 09:00, 81.2 ng/mL at 12:31, and 58 ng/mL at 16:50 which were deemed not clinically significant indicators of cardiac ischemia.
The patient’s EKG findings resolved within 30 minutes of TXA discontinuation, and he reverted to normal sinus rhythm (Figure 1) with no evidence of aberrations. At the time of development of the LBBB (Figure 2 and Figure 3), the patient denied any chest discomfort, dyspnea, diaphoresis, nausea, vomiting, or other clinical manifestations suggestive of acute coronary syndrome. The patient’s hemoptysis continued, and a second round of inhaled TXA was administered 8 hours later, during which the LBBB (Figure 4) similarly resolved in approximately 30 minutes (Figure 5). The medication was discontinued shortly after the second event. Hemoptysis did not recur after second TXA administration.
DISCUSSION
Given the lack of troponin elevation and the absence of symptoms characteristic of acute coronary syndrome, a primary coincident cardiac event being the cause of the LBBB is unlikely. In addition, the EKG performed during the active LBBB did not meet the Sgarbossa criteria [4] for a myocardial infarction during a LBBB (including a concordant ST elevation >1 mm in leads with a positive QRS, concordant ST depression >1 mm in V1–V3, and/or excessively discordant ST elevation >5 mm in leads with a negative QRS complex). However, the resolution of the LBBB soon after drug discontinuation and the reproducibility of this event by a second dose of inhaled TXA increases the probability that it is indeed due to the direct effects of the drug itself. Only recently has inhaled TXA been seen as a modality for the treatment of massive (>200 mL/24 hour) and non-massive hemoptysis [1],[2], particularly for cancer-induced or bronchiectasis-induced hemoptysis [2]. As a result, few studies have been conducted on the therapeutic efficacy of inhaled TXA and the side effect profile of its locally administered form [3].
Studies have reported local application of TXA to be effective in controlling bleeding of different etiologies [5]. In reference to our patient, inhalational TXA effectively controlled his hemoptysis after only two doses. This was especially beneficial in his case given his multiple comorbidities and his high risk for systemic thromboembolism in the setting of a recent embolic stroke, recurrent DVT and PE, and a positive lupus anticoagulant. Local administration of TXA at the site of action allows therapeutic drug concentration to be achieved with a shorter interval as compared to systemic administration. One study found topical TXA applied to patients’ oral cavities achieved therapeutic concentrations within 30 minutes and remained so for more than 2 hours. The same study found oral administration of TXA to have failed achieving a detectable level in the saliva despite a high mean plasma drug concentration; emphasizing the efficacy of topical TXA compared its oral counterpart in selected clinical scenarios [6]. This, in turn, may support the use of the local form of TXA in patients with a hypercoagulable predisposition.
Our case sheds light on the need for further investigation on the cardiac safety of inhaled TXA. It also necessitates the need for comparing side effect profiles based on different routes of administration. Oral TXA adverse effects, according to data from clinical trials and post-marketing surveillance conducted by the FDA, include seizures, headaches, backache, and abdominal pain [3]. To the best of our knowledge, in the setting of oral TXA use, only three cases of myocardial infarction have been reported [7]. One of these three cases had concomitant use of intravenous desmopressin (DDAVP) with TXA in a patient with desmopressin responsive platelet dysfunction [8],[9]. Development of myocardial infarction in such a setting makes differentiating the possible contribution of pain, nausea, vomiting, diarrhea, fatigue, pulmonary embolism, deep vein thrombosis, anaphylaxis, impaired color vision, and other visual disturbances DDAVP from that of TXA difficult. Reported side effects with inhaled TXA only include bronchospasm, and no cases of myocardial ischemia/infarction have been reported thus far [3].
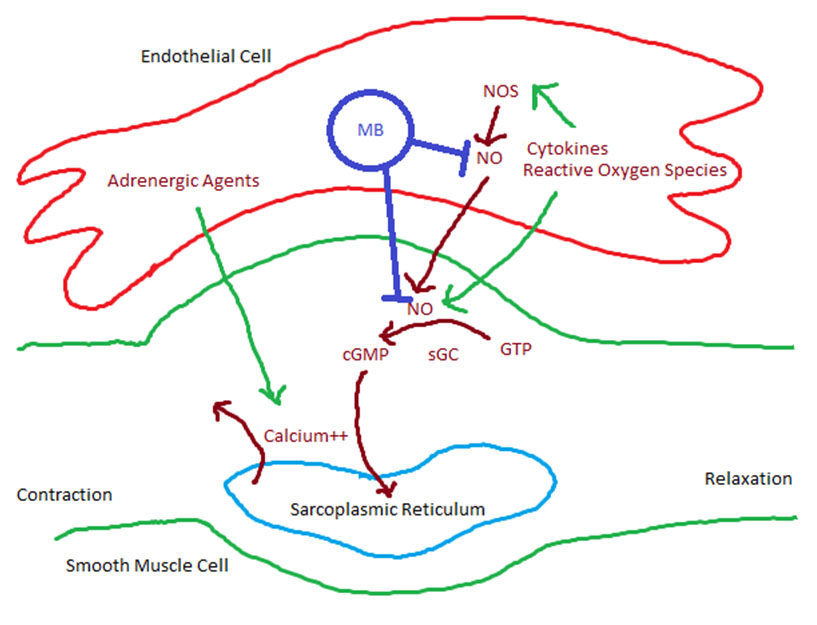
The pathophysiological cause for the arrhythmic effect of TXA to cause a LBBB is currently unknown. An experimental paper in 2009 noted L-lysine, with structural analogy to TXA, increased the force of atrial contraction. Furthermore, TXA exhibited an inhibitory effect on serotonin’s inotropic cardiac effect through antagonistic binding to the 5HT4 channel in the human right atrium [10]. Therefore, L-lysine can be implicated in arrhythmias originating from the atrium, such as atrial fibrillation. To our knowledge, however, its role in causing conduction defects or arrhythmias originating below the AV node, particularly the His-Purkinje system, is unclear. Therefore, further studies on the pathophysiological effect of TXA on the human heart and its conduction system may prove to be beneficial.
CONCLUSION
Inhaled TXA can be associated with cardiac effects and thus cardiac monitoring during TXA administration is recommended. Side effects can be identified by recognizing symptoms, eliciting signs, telemetry/electrocardiogram, and measuring cardiac biomarkers and therefore caution should be exercised in the administration of TXA, especially in the use of inhaled TXA with patients who have concomitant cardiovascular disease. Patients with underlying cardiac conduction abnormalities such as first degree AV delay, right or left BBB should be closely monitored given potential risk of complete heart block. Prospective trials and case reports will bring further clarity regarding risks versus benefits in the use of inhaled TXA.
REFERENCE
1.
Komura S, Rodriguez RM, Peabody CR. Hemoptysis? Try inhaled tranexamic acid. J Emerg Med 2018;54(5):e97–9. [CrossRef]
[Pubmed]

2.
Wand O, Guber E, Guber A, Shochet GE, Israeli-Shani L, Shitrit D. Inhaled tranexamic acid for hemoptysis treatment: A randomized controlled trial. Chest 2018;154(6):1379–84. [CrossRef]
[Pubmed]

3.
4.
Sgarbossa EB, Pinski SL, Barbagelata A, et al. Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle-branch block. GUSTO-1 (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) Investigators. N Engl J Med 1996;334(8):481–7. [CrossRef]
[Pubmed]

5.
González-Castro A, Rodriguez-Borregán JC, Chicote E, Escudero P, Ferrer D. Nebulized tranexamic acid as a therapeutic alternative in pulmonary hemorrhage. [Article in Spanish]. Arch Bronconeumol 2018;54(8):442–3. [CrossRef]
[Pubmed]

6.
Sindet-Pedersen S. Distribution of tranexamic acid to plasma and saliva after oral administration and mouth rinsing: A pharmacokinetic study. J Clin Pharmacol 1987;27(12):1005–8. [CrossRef]
[Pubmed]

7.
Sirker A, Malik N, Bellamy M, Laffan MA. Acute myocardial infarction following tranexamic acid use in a low cardiovascular risk setting. Br J Haematol 2008;141(6):907–6. [CrossRef]
[Pubmed]

8.
Mekontso-Dessap A, Collet JP, Lebrun-Vignes B, Soubrié C, Thomas D, Montalescot G. Acute myocardial infarction after oral tranexamic acid treatment initiation. Int J Cardiol 2002;83(3):267–8. [CrossRef]
[Pubmed]

9.
Iacobellis G, Iacobellis G. Combined treatment with tranexamic acid and oral contraceptive pill causes coronary ulcerated plaque and acute myocardial infarction. Cardiovasc Drugs Ther 2004;18(3):239–40. [CrossRef]
[Pubmed]

10.
Boldt A, Gergs U, Frenker J, et al. Inotropic effects of L-lysine in the mammalian heart. Naunyn Schmiedebergs Arch Pharmacol 2009;380(4):293–301. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Acknowledgement
We would like to thank Dr. Peter L Larsen for providing our team with guidance and editorial support.
Author ContributionsKatharine Lasley Woods - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Sanjana Nagraj - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Nicholas Germano - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ketan Koranne - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Douglas T Summerfield - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2020 Katharine Lasley Woods et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.