|
Case Series
Polymyxin B hemoperfusion in septic patients with acute myeloblastic and premyelocytic leukemia
1 UO Anestesia e Rianimazione Santa Chiara, Azienda Ospedaliero-Universitaria Pisana, Via Roma 67, 56126 Pisa, Italy
Address correspondence to:
Luigi De Simone
UO Anestesia e Rianimazione Santa Chiara, Azienda Ospedaliero-Universitaria Pisana, Via Roma 67, 56126 Pisa,
Italy
Message to Corresponding Author
Article ID: 100094Z06LS2021
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
De Simone L, Valentini K, Maceri F, Cidin S, Benedetti E, Del Pellegrino V, Paladini AM. Polymyxin B hemoperfusion in septic patients with acute myeloblastic and premyelocytic leukemia. Case Rep Int 2021;10:100094Z06LS2021.ABSTRACT
Introduction: Infections are frequent complications in patients with malignant hematological disease and are associated with high morbidity and mortality. The use of polymyxin B hemoperfusion (PMX-HP) as an additional therapy in hematological patients who develop sepsis or septic shock following chemo- or immunosuppressive therapy is not often described in literature.
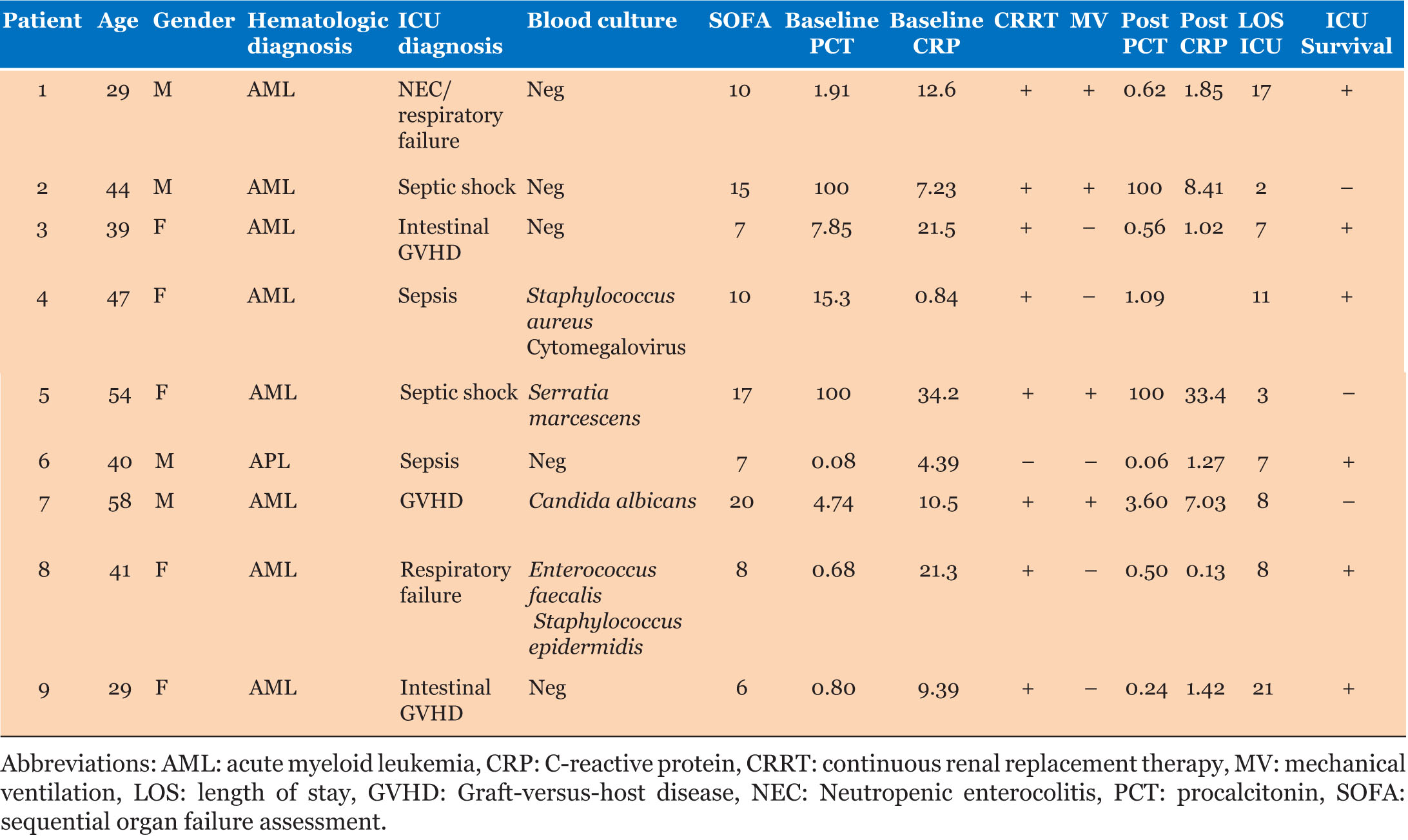
Case Series: This study included patients with hematological neoplasms (acute myeloblastic or promyelocytic leukemia) who were undergoing chemotherapy (pre- or post-transplant) and developed sepsis or septic shock requiring organ support. The patients received one session of polymyxin B hemoperfusion generally followed by at least 72 hours of continuous renal replacement therapy (CRRT) as renal support. The clinical condition, hemodynamic parameters, and blood chemistry values of the patients were monitored until discharge from the intensive care unit (ICU). Nine patients were included in this study. Of the 9 patients, 6 survived and were discharged from the ICU. In the 6 surviving patients a drastic reduction in procalcitonin (PCT) and C-reactive protein (CRP) were observed after treatment with PMX-HP. No adverse events related to the PMX-HP treatment occurred.
Conclusion: Patients with hematological neoplasms complicated with septic shock have a very high mortality. In our experience, polymyxin B hemoperfusion therapy and CRRT in addition to conventional therapy may be a valid strategy to improve outcome in this type of patients.
Keywords: Acute myeloblastic leukemia, Infection, Polymyxin B hemoperfusion, Promyelocytic leukemia, Sepsis, Septic shock
INTRODUCTION
Infections are frequent complications in patients with malignant hematological disease and are associated with high morbidity and mortality and consequently influence on quality of life and hospital costs [1].
Intensive anti-neoplastic chemotherapy protocols, new classes of drugs (monoclonal antibodies and hypomethylating agents) and the increase in allograft procedures have resulted in an increase in toxicity and infectious risk [1]. Chemotherapy represents one of the most frequent causes of acute tumor lysis syndrome (ATLS) due to the rapid separation of neoplastic cells and consequent release of their contents into the circulation. This condition can be associated with various clinical conditions, including infection and sepsis [2].
In the past 30 years, the etiology of bacteremia in neutropenic patients has undergone changes with a significant increase in Gram-negative bacteria as a cause of bacteremia [2]. In sepsis caused by Gram-negative bacteria, circulating endotoxin plays a fundamental role as the most powerful trigger of the septic cascade and may progress to multiple organ dysfunction syndrome (MODS) and death [3].
According to the Surviving Sepsis Campaign guidelines [4], early diagnosis, aggressive resuscitation, adequate antibiotic therapy, source control, and organ support are the key elements of sepsis management. However, conventional therapy is not always sufficient and therefore different treatments, alone or combined, can be used to obtain a better immune response. Polymyxin B hemoperfusion therapy (PMX-HP) is able to remove up to 90% of circulating endotoxin and also represents several immunomodulating mechanisms resulting from the direct adsorption of inflammatory mediators, immune cells, and circulating apoptotic factors [5],[6]. It has been reported that PMX-HP contributes to the decrease in expression and inhibition of the release of HMGB-1 [7].
Excessive conditioning regimens such as total body irradiation and chemotherapy deteriorate the host tissue. In turn, damaged cells secrete cytokines and other damage-associated molecular patterns (DAMPs). These factors trigger or intensify graft-versus-host disease (GVHD) and influence adaptive immunity by contributing to the activation of immune cells such as alloreactive T cells [8]. Intranuclear HMGB-1 released from immune cells and necrotic cells damaged by TNF-α and IL-6 acts as a DAMP that accelerates immune and inflammatory responses, activates innate immunity, and acts as a mediator of various pathological conditions such as acute rejection by allograft and septic syndromes [9]. Therefore, HMGB-1 could represent a potential molecular target for GVHD therapies and its removal represents a determining factor on the outcome of these patients [10].
In addition, PMX-HP may play a role in reducing mortality in patients with sepsis or septic shock [11] and seems to aid in restoring hemodynamics and respiratory function [12].
Based on this rationale, we decided to use PMX-HP as an adjunctive therapy in patients affected by acute myeloid leukemia (AML) with confirmed or suspected infective status. Since an infective status can be deleterious in patients affected by leukemia undergoing chemotherapy or pre-/post-transplant conditioning due to the lack of immunocompetence. Our clinical strategy was to minimize the risks associated with severe infection, by neutralizing the trigger of infection and the inflammatory cascade. This is to minimize the impact of septic shock or to try to avoid its development, to preserve availability to transplant or to preserve the graft in the post-transplant phase.
Case Series
The PMX-HP cartridge (Toraymyxin, Toray Medical, Japan) is a medical device designed to perform hemoperfusion therapy with polymyxin B in order to neutralize endotoxin present in the patient’s blood. Toraymyxin consists of a cartridge with polystyrene/polypropylene fibers to which polymyxin B has been covalently immobilized [13]. One cycle of 2 hours of hemoperfusion was performed using a blood flow rate of 100 mL/min and unfractionated heparin was used as anticoagulant.
We treated 9 patients diagnosed with sepsis or septic shock (4 males and 5 females) aged from 29 to 58 years and affected by acute myeloblastic or promyelocytic leukemia undergoing chemotherapy or pre-/post-transplant conditioning. Blood samples for cultures were drawn immediately before the patients were admitted to the ICU. Table 1 shows baseline and clinical characteristics of the 9 patients before and after treatment with PMX-HP. Of the 9 patients, 6 survived and were discharged from the ICU. In the 6 surviving patients a drastic reduction in PCT and CRP was observed after treatment with PMX-HP. During PMX-HP treatment there were no episodes of filter coagulation, bleeding, or metabolic complications (e.g., hypocalcemia/hypercalcemia or metabolic disorders). No adverse events occurred and the PMX-HP treatment was safe.
DISCUSSION
The use of polymyxin B hemoperfusion as an additional therapy in hematological patients who develop sepsis or septic shock following chemo- or immunosuppressive therapy is not often described in literature. One observational study investigated the use of PMX-HP in 16 patients with hematological disease and Gram-negative septic shock [14]. In this study serum endotoxin concentration was measured and was found to be consistently elevated in all patients at baseline. Ten out of 16 patients did not survive, but no data on clinical parameters collected after treatment were reported.
In the present study, we have treated 9 patients with PMX-HP in addition to conventional therapy and CRRT sequentially was performed as for renal support when needed. Of the 6 patients who survived to ICU discharge, both PCT and CRP were reduced drastically after treatment with PMX-HP. Treatment with PMX-HP, although not a curative therapy for the onco-hematological disease itself, may limit or modulate the complications associated with it. An explanation for this may be found in the immunomodulatory effects of the treatment. We may hypothesize that PMX-HP therapy provides the time needed for the bone marrow to resume its function. Although the PMX-HP cartridge was designed to adsorb endotoxin, other mechanisms of immunomodulation have been demonstrated. Some of these mechanisms are caused by the elimination of endotoxin, while others result from the direct adsorption of activated monocytes [15], activated neutrophils as well as reducing the levels of circulating apoptotic factors [5].
CONCLUSION
Since an infective status can be deleterious in patients affected by leukemia undergoing chemotherapy or pre-/post-transplant conditioning due to the lack of immunocompetence, our clinical strategy was to minimize the risk of severe infection, by neutralizing the infection trigger and the inflammatory cascade. This is to minimize the impact of septic shock when present or to try to avoid its development, to preserve availability to transplant or to preserve the graft in the post-transplant phase.
Sepsis and septic shock are time-dependent conditions, the clinical outcome is strictly correlated with the timing of diagnosis and effectiveness by which the disease is managed.
Our clinical experience suggests that PMX-HP can be a useful adjuvant therapy for the management of patients affected by AML with confirmed or suspected infective status.
Further clinical investigation including randomized clinical trials is needed to confirm the effectiveness of PMX-HP in this particular type of patients.
REFERENCE
1.
McArdle JR. Critical care outcomes in the hematologic transplant recipient. Clin Chest Med 2009;30(1):155– 67, ix–x. [CrossRef]
[Pubmed]

2.
Ferrà C, Marcos P, Misis M, et al. Outcome and prognostic factors in patients with hematologic malignancies admitted to the intensive care unit: A single-center experience. Int J Hematol 2007;85(3):195–202. [CrossRef]
[Pubmed]

3.
Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013;369(9):840–51. [CrossRef]
[Pubmed]

4.
Rhodes A, Evans E, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43(3):304–77. [CrossRef]
[Pubmed]

5.
Cantaluppi V, Assenzio B, Pasero D, et al. Polymyxin-B hemoperfusion inactivates circulating proapoptotic factors. Intensive Care Med 2008;34(9):1638–45. [CrossRef]
[Pubmed]

6.
Ronco C, Klein DJ. Polymyxin B hemoperfusion: A mechanistic perspective. Crit Care 2014;18(3):309. [CrossRef]
[Pubmed]

7.
Srisawat N, Tungsanga S, Lumlertgul N, et al. The effect of polymyxin B hemoperfusion on modulation of human leukocyte antigen DR in severe sepsis patients. Crit Care 2018;22(1):279. [CrossRef]
[Pubmed]

8.
Maeda Y. Pathogenesis of graft-versus-host disease: Innate immunity amplifying acute alloimmune responses. Int J Hematol 2013.98(3):293–9. [CrossRef]
[Pubmed]

9.
Wang H, Liao H, Ochani M, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med 2004;10(11):1216–21. [CrossRef]
[Pubmed]

10.
Im KI, Kim N, Lim JY, et al. The free radical scavenger NecroX-7 attenuates acute graft-versus-host disease via reciprocal regulation of Th1/regulatory T cells and inhibition of HMGB1 release. J Immunol 2015;194(11):5223–32. [CrossRef]
[Pubmed]

11.
Zhou F, Peng Z, Murugan R, Kellum JA. Blood purification and mortality in sepsis: A meta-analysis of randomized trials. Crit Care Med 2013;41(9):2209– 20. [CrossRef]
[Pubmed]

12.
Cruz DN, Antonelli M, Fumagalli R, et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: The EUPHAS randomized controlled trial. JAMA 2009;301(23):2445–52. [CrossRef]
[Pubmed]

13.
14.
Mori M, Onaka T, Yonezawa A, Kitagawa T, Sasaki Y, Imada K. Direct hemoperfusion using polymyxin-B immobilized fiber for severe septic patients with hematological disorders: A single-center analysis. J Infect Chemother 2014;20(4):282–4. [CrossRef]
[Pubmed]

15.
Nishibori M, Takahashi HK, Katayama H, et al. Specific removal of monocytes from peripheral blood of septic patients by polymyxin B-immobilized filter column. Acta Med Okayama 2009;63(1):65–9. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Luigi De Simone - Conception of the work, Design of the work, Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Katia Valentini - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Francesca Maceri - Acquisition of data, Analysis of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Sabrina Cidin - Acquisition of data, Analysis of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Edoardo Benedetti - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Valentina Del Pellegrino - Acquisition of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Anna Maria Paladini - Acquisition of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2021 Luigi De Simone et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.