|
Case Report
Beyond Good’s syndrome: A case of multifactorial thymoma-related immunodeficiency with cytomegalovirus reactivation
1 MD, Medical Resident, Department of Infectious Diseases, Hospital de Santa Maria at Centro Hospitalar Universitário Lisboa Norte, Lisbon, Portugal
2 MD, Attending Physician, Department of Infectious Diseases, Hospital de Santa Maria at Centro Hospitalar Universitário Lisboa Norte, Lisbon, Portugal
3 MD, Attending Physician and Head of Department, Department of Infectious Diseases, Hospital de Santa Maria at Centro Hospitalar Universitário Lisboa Norte, Lisbon, Portugal
Address correspondence to:
Maria Cunha
Hospital de Santa Maria, Serviço de Doenças Infecciosas, Avenida Professor Egas Moniz, 1649-035 Lisboa, Lisbon,
Portugal
Message to Corresponding Author
Article ID: 100095Z06MC2021
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Cunha M, Leitão I, Marques T, Pereira Á. Beyond Good’s syndrome: A case of multifactorial thymoma-related immunodeficiency with cytomegalovirus reactivation. Case Rep Int 2021;10:100095Z06MC2021.ABSTRACT
Introduction: Thymomas, by virtue of T-cell maturation derangement, are frequently accompanied by parathymic syndromes. Classically, Good’s syndrome characterizes immunodeficiency in patients with thymoma, comprising of hypogammaglobulinemia and various degrees of T-cell impairment; however, these patients frequently suffer from additional iatrogenic effects of chemotherapy or immunosuppressors used to control autoimmune phenomena. As a result, they often exhibit signs of decreased bone marrow response and cytopenias, leading to opportunistic infections such as cytomegalovirus (CMV) reactivation, whose role in perpetuating the state of immunodeficiency may be under-explored.
Case Report: We describe the case of a 62-year-old man with a thymoma who exhibited several paraneoplastic syndromes, including Good’s syndrome. The patient had also been diagnosed with sigmoidal adenocarcinoma, previously medicated with capecitabine and was currently medicated with corticosteroids and azathioprine, placing an additional strain on his already compromised immune system. After being inadvertently exposed to the combination of azathioprine and allopurinol, he developed pancytopenia with little response to granulocyte-colony stimulating factor (G-CSF) after withdrawal of the offending agents. A high CMV viral load was suspected as a cause for perpetuating leukopenia. Treatment with ganciclovir resulted in bone marrow recovery, and immunoglobulin replacement together with antibiotic and antiviral prophylaxis prevented other serious infections until thymectomy and completion of chemotherapy.
Conclusion: This case exemplifies many of the complexities in managing patients with thymomas, particularly when balancing autoimmunity and immune suppression. Cytomegalovirus reactivation is not unusual in this context and cytopenias may be the only manifestation. Although treatment of symptomatic disease is beneficial, the role of prophylaxis is not yet consensual due to its potentially myelosuppressive effects.
Keywords: Cytomegalovirus, Good’s syndrome, Immunodeficiency, Thymoma
INTRODUCTION
Thymomas are rare tumors of the mediastinum derived from the thymic epithelium. The thymus is the site of maturation for T cells, playing a central role in the development of immunocompetent T cells, differentiation and proliferation of T-cell subsets, the evolution of naive T-cells into T helper cells (CD4), and cytotoxic suppressor cells (CD8), and migration of mature T cells into the circulating lymphocyte pool and peripheral tissues. The process of immune system maturation is most active during childhood; indeed, thymus growth peaks around the ages of two or three and begins to shrink during adolescence. Although some activity is retained during adulthood, involution of the thymus partially accounts for increased susceptibility to infections during old age [1]. Adequate functioning of the thymus is not only crucial for achieving protection against infectious agents, but also in induction of immune self-tolerance in order to prevent self-harm or autoimmunity [2]. Deregulation of thymopoiesis via decreased thymic epithelial expression of the thymic autoimmune regulator gene (AIRE) and major histocompatibility complex (MHC) Class II, as well as alterations in thymic architecture lead to autoimmune complications that manifest as paraneoplastic disorders [3].
Over 20 parathymic syndromes have been described, affecting approximately 40% of patients with thymoma [4]. A particular challenge to physicians treating patients with thymoma is the overlapping of autoimmunity with immunosuppression, often aggravated by treatment of parathymic syndromes, coexisting neoplastic diseases and unintended iatrogenic effects [5].
Immunodeficiency characterized by hypogammaglobulinemia with variable rates of T-cell derangement affects 6–11% of patients with thymoma and is usually referred to as Good’s syndrome. This parathymic syndrome usually presents in the 4th or 5th decade of life, affecting both men and women at similar rates, and is typically diagnosed following an infectious complication [6].
According to one case series of 21 patients with Good’s syndrome, capsulated organisms such as Streptococcus pneumoniae and Haemophilus influenzae are the most frequent cause of infection, with 86% of patients reporting pneumonia, 57% reporting bronchiectasis, and 33% developing sinusitis. Chronic and recurring diarrhea are also an important feature, affecting 38% of patients; opportunistic infections associated with cellular immune defects seem to be less prevalent, but may include sporadic cases of mucosal candidiasis, aspergillosis, isosporosis, disseminated infection by Mycobacterium tuberculosis and cytomegalovirus (CMV) disease such as colitis and retinitis [6],[7].
In this context, management of CMV reactivation represents the ultimate trial, since both this opportunistic infection and its treatment may result in further damage to an already fragile immune system.
CASE REPORT
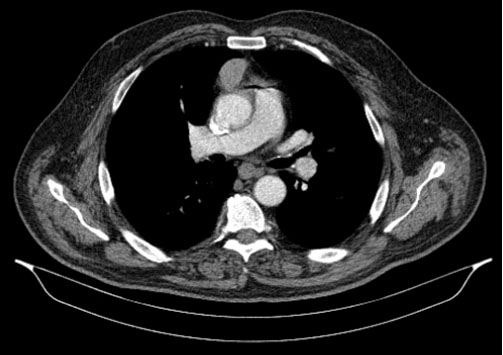
We describe the case of a 62-year-old man who was referred to an infectious diseases consultation on January 14, 2019 with the presumptive diagnosis of chronic facial impetigo. The patient had a history of myasthenia gravis diagnosed 9 months earlier, when he presented with ptosis, dysphagia and fatigue, and was positive for acetylcholine receptor antibodies. During the following investigation, a computer tomography (CT) scan detected a 5 cm mass in the anterior mediastinum suggestive of thymoma (Figure 1). He was scheduled for a surgical intervention in February 2019. He was also diagnosed with a grade-1 sigmoidal adenocarcinoma, having been subjected to sigmoidectomy six months prior to the consultation. He had also been treated with one cycle of capecitabine, which had been suspended due to gastrointestinal and hematological intolerance.
At the time, he was medicated with 100 mg azathioprine, 40 mg prednisolone, pyridostigmine 5 id and omeprazole. Following a diagnosis of corticoid-associated Diabetes Mellitus, he was started on oral antidiabetic medication. He described several courses of antibiotics for his skin condition, with no relief.
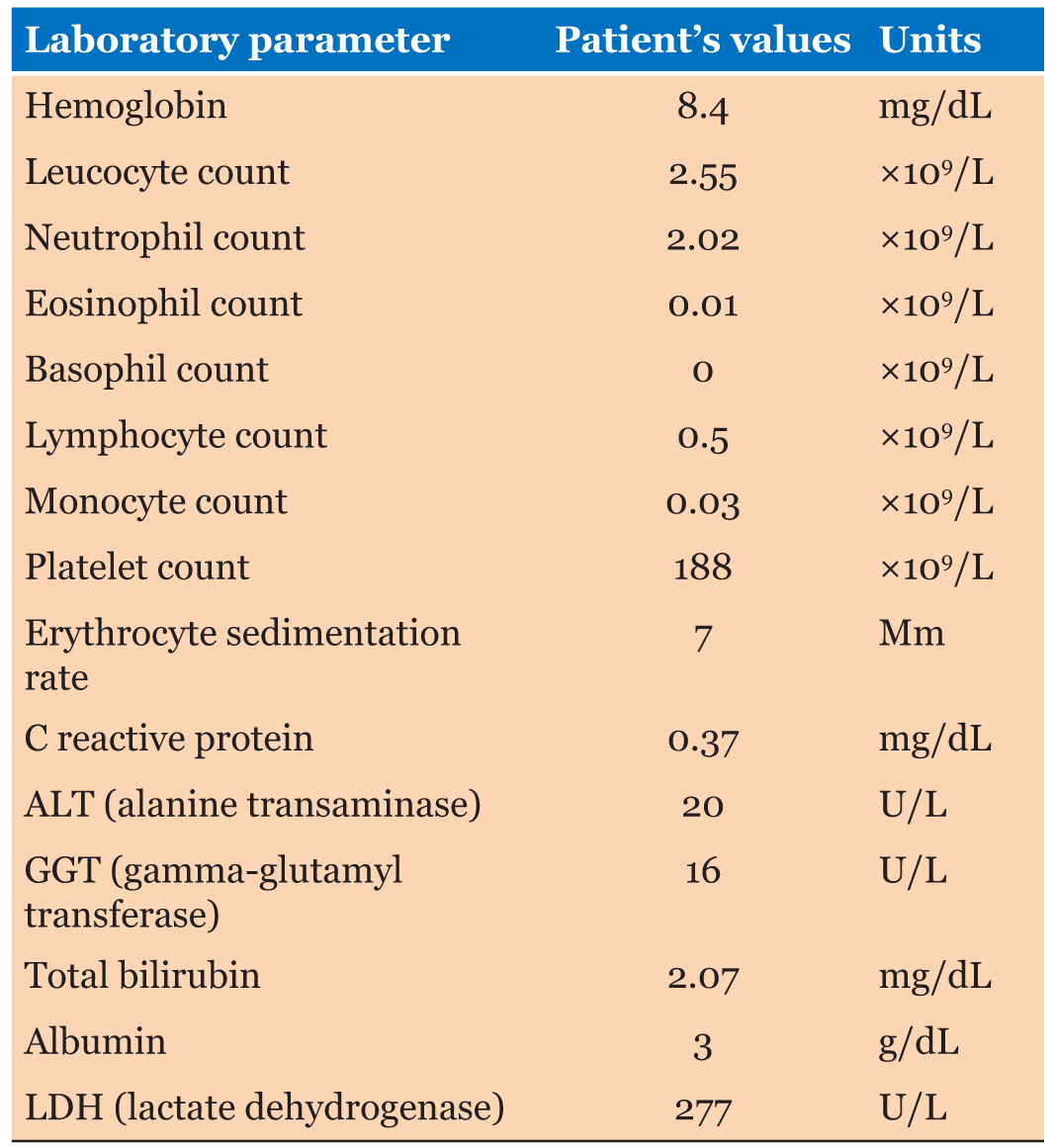
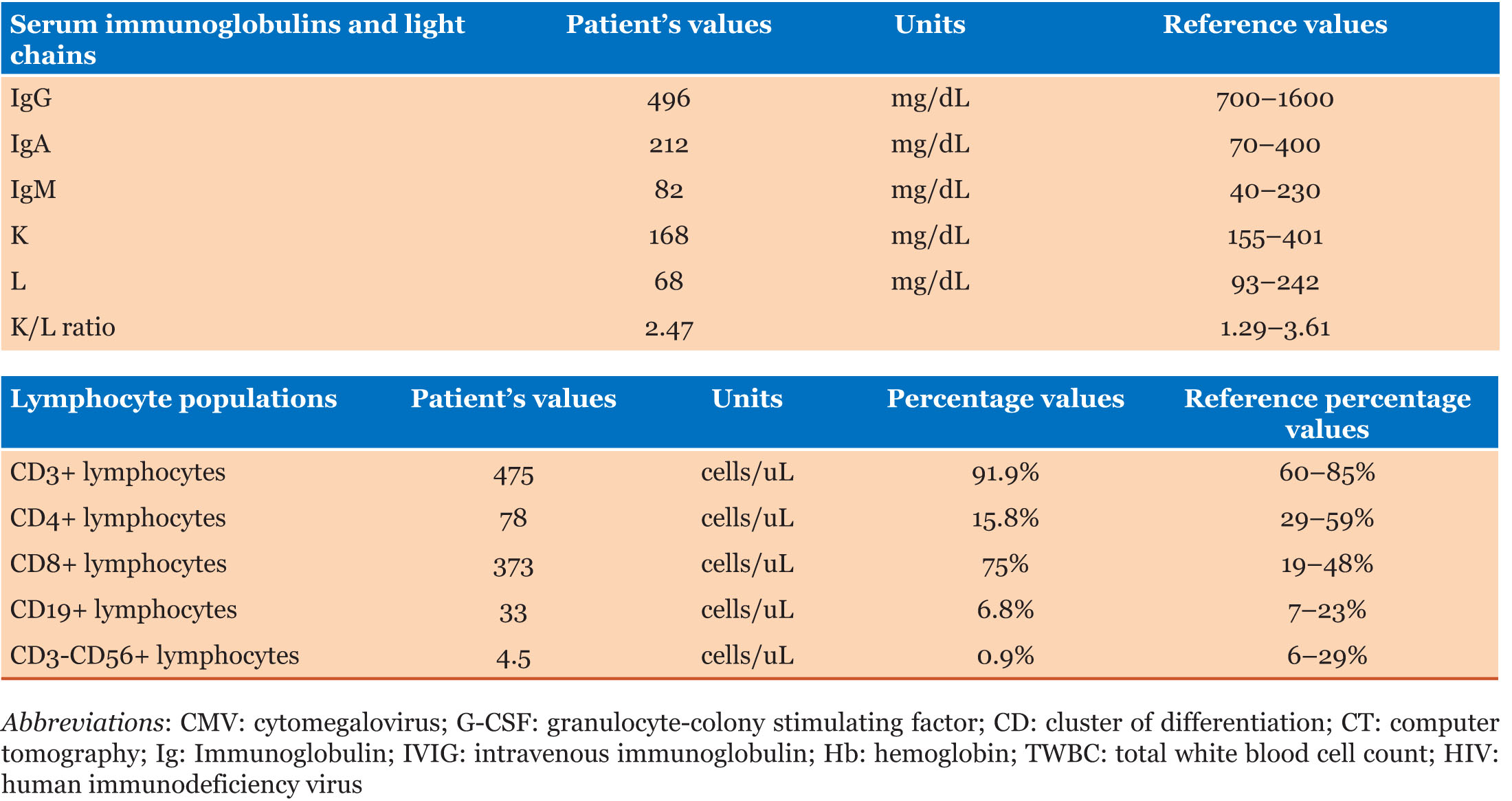
On physical examination, he was found to have flaccid blisters and erosions on his face and neck (Figure 2A). Blood tests revealed bicytopenia, with low hemoglobin as well as total white blood cell count, including CD4+T-cell and B cell lymphopenia, as well as hypogammaglobulinemia, with a normal erythrocyte sedimentation rate and C reactive protein (Table 1 and Table 2).
Considering the more likely possibility of the skin lesions representing pemphigus in the setting of thymoma-related autoimmunity, the patient was submitted to a skin biopsy (Figure 3) (immunofluorescence revealed IgG and complement reticular deposits in epidermis with acantholysis) and proposed for intravenous immunoglobulin (IVIG) treatment. Paraneoplastic pemphigus serum antibody screening was negative.
The co-existence of thymoma-related immunodeficiency (Good’s syndrome) was also considered, regarding the new-onset hypogammaglobulinemia as well as low B and CD4+ T cell count. Considering his low immunity status, he was started on a prophylactic dose of cotrimoxazole.
Two days later, he was admitted to the Emergency Room complaining of severe fatigue and was found to be hypotensive (blood pressure 81/48 mmHg). His skin lesions now showed yellow crusting. Blood tests revealed a further drop in hemoglobin (Hb 6.5 mg/dL) and leukocytes (TWBC 1.06 × 109/L). Considering his rapidly declining general state, he was admitted to the Infectious Diseases ward of the Santa Maria University Hospital.
Upon reviewing the patient’s medical history, it was discovered that he had recently been prescribed allopurinol for hyperuricemia (uricemia 9.7 mg/dL, no clinical picture of gout) by his general physician. Treatment with azathioprine and allopurinol was immediately suspended considering the interaction between these drugs as a likely aggravating factor for the bicytopenia. He was given a blood transfusion and started on darbepoetin, folic acid, granulocyte-colony stimulating factor (G-CSF), and intravenous immunoglobulin (IVIG). He was also medicated with flucloxacillin for likely staphylococcal superinfection of pemphigus lesions.
Hypotension eventually responded to fluids, antibiotics and to lowering the dose of acetylcholinesterase inhibitors. After a 5-day course of immunoglobulin he was restarted on azathioprine.
Despite these measures, the patient remained severely anemic, requiring 2 additional blood transfusions. He had no evidence of blood loss or hemolysis and had a reticulocyte index of 0.5. Erythropoietin levels were above reference values (34.4 mIU/mL). Considering the possibility of thymoma-related pure red cell aplasia, a myelogram was preformed, revealing a myeloid/erythroid ratio of 1:1.8, with increased iron deposits and no evidence of dysplasia.
Following a slower than expected leukocyte count rise and the development of thrombocytopenia, a CMV viral load was ordered and was found to be 428,920.3 copies/mL. He had no other target organ involvement and was started on ganciclovir.
After treating a nosocomial bacteremia to Serratia marcescens with ertapenem (isolated in 2 sets of hemocultures taken 12 days after admission; previous blood cultures taken 5 days earlier were negative), following 10 days of ganciclovir, the patient’s leukocyte and platelet count normalized. Hemoglobin stabilized at 10 g/dL under darbepoetin. Twenty-three days after being admitted to the hospital, he was subjected to surgical thymoma excision. Pathology confirmed B3 thymoma with transcapsular invasion Masaoka stage IIa, with atypical glandular structures. After a multidisciplinary discussion, it was decided to initiate adjuvant chemotherapy (cisplatin and etoposide) as well as local radiotherapy. The patient was discharged under once weekly darbepoetin, valganciclovir 900 mg 2 id, azathioprine 100 mg 1 id, prednisone 30 mg 1 id, thrice weekly cotrimoxazole 960 mg, pyridostigmine 60 mg 3 id, omeprazole 20 mg, gliclazide 30 mg 1 id and metformine/vildagliptine 1 id.
One month after thymectomy, his T-lymphocyte CD4+ counts had normalized at 529 cells/uL and IgG levels were now 759 mg/dL. Cytomegalovirus viral load remained under 200 UI/L. Darbepoetin was stopped at this time.
Corticosteroids were slowly weaned off over a 6-month period and prophylactic medications were discontinued after completing chemo and radiotherapy cycles.
One year after first being admitted to the hospital, the patient exhibits complete resolution of skin lesions (Figure 2B), is asymptomatic, has a slight anemia (Hb 12.7 g/dL) and an otherwise normal blood cell count.
Follow-up CT-scans reveal no evidence of tumor relapse. Pyridostigmine has been suspended and he remains medicated with azathioprine.
DISCUSSION
Immunodeficiency in these patients is often multifactorial, encompassing thymoma-related paraneoplastic immunodeficiency (Good’s syndrome) as well as iatrogenic and infectious complications.
Good’s syndrome and thymoma-related immunodeficiency
The principal immunological findings in Good’s syndrome are hypogammaglobulinemia, few or absent B cells, an abnormal CD4+:CD8+ T cell ratio, CD4 T cell lymphopenia, and impaired T cell mitogenic responses [6],[7]. NK-cell loss also seems to contribute to the increased infection risk [8]. Although less frequent, clinically relevant T-cell immunodeficiency can also occur in the absence of hypogammaglobulinemia [9].
Disturbance of both humoral and cellular components leads to a high risk of infectious complications, often by atypical or opportunistic agents, resulting in high mortality rates, from 44.5% to 57%, according to different sources [10].
Although there is currently no consensual explanation for B-cell deficiency and hypogammaglobulinemia in these patients, a few mechanisms have been explored, such as a cytokine or T-cell-mediated inhibition of B-cell growth and differentiation in the bone marrow [11]. It has recently been proposed that neutralizing autoantibodies against multiple cytokines may be acting in concert to impair host defense, although their direct role in disease causation remains to be definitively established [12].
Iatrogenic immunosuppression: treatment of autoimmune manifestations and neoplasms
Immune system derangement in the presence of Good’s syndrome increases the probability of developing other autoimmune disorders. A recently published article analyzing the clinical and laboratory features of 78 United Kingdom patients with Good’s syndrome documented the presence of autoimmunity in 26% of patients, with pure red cell aplasia in 10% and myasthenia gravis in 4% [13]. A 2017 systematic review examining the characteristics of 47 Chinese patients similarly described autoimmunity in 36% of patients with Good’s syndrome, with 17% pure red cell aplasia and 6% myasthenia gravis [14].
Pure red cell aplasia carries a high rate of relapse even after thymoma resection, frequently requiring adjuvant immunosuppressive therapy [6],[15]. Similarly, myasthenia gravis often requires corticosteroid treatment, other than cholinesterase inhibition. Furthermore, because of the significant adverse effects of corticosteroids and treatment resistance, many patients will require additional therapy such as azathioprine, mycophenolate, or tacrolimus [16].
Immunosuppressive therapy imposes an additional challenge in infection prevention and treatment, and it is therefore recommended that these patients be followed-up by an experienced clinician. Patients and their other attending physicians must be aware of thymoma diagnosis as well as recent and current treatments in order to avoid unintended iatrogenic effects such as the one suffered by the patient presented above, who was simultaneously treated with azathioprine and allopurinol. Xanthine oxidase inhibitors blunt the degradation of 6-mercaptopurine, resulting in the accumulation of active metabolites that are known to decrease replication and activation of leukocytes while also inducing apoptosis, potentially resulting in serious bone marrow aplasia [17].
Immunologic derangements related to thymoma also increase the possibility of developing secondary neoplasms, often arising concurrently with autoimmune manifestations. Various sources place the incidence of a secondary neoplasm in thymoma patients as being around 16%, ranging from 8% to 31% [18]. In this setting, treatment of both the disorders must be carefully coordinated, considering the increased risks of chemotherapy and surgery in these patients [19].
Opportunistic infections: the CMV conundrum
The aforementioned combined immunodeficiency state becomes fertile ground for opportunistic infections; one example includes a case in which a patient developed CMV enteritis following immunosuppressive treatment with azathioprine and steroids resulting in therapy-induced leukopenia [20]. It has recently been shown that patients with Good’s syndrome are particularly susceptible to CMV-mediated disease, even in the presence of significantly greater T cell numbers than what is seen in other immune disorders such as human immunodeficiency virus (HIV) infection. In fact, CMV reactivation has been documented in the presence of normal CD4+ T cell counts [21].
Although CMV reactivation may result in a wide array of manifestations, with documented involvement of retina, gastrointestinal tract, central nervous system, lungs [22] and even thymoma cells [23], little attention has been given to its role in aggravating immune depletion. While CMV has long been considered a “usual culprit” of cytopenia in hematological and transplant patients, it is often overlooked in other states of immunosuppression. As an example, we cite the case of 42-year-old man with metastatic thymoma who suffered from several opportunistic diseases and was found to have CMV viremia without evidence of tissue-invasive disease. He received 2 weeks of induction-dose intravenous ganciclovir, which eventually resulted in viremia clearance but was complicated by pancytopenia, likely multifactorial [24]. The possibility that CMV viremia might have contributed to pancytopenia in this patient was not discussed and blood cell counts at the time CMV viremia was detected were not presented.
Despite the clear link between CMV reactivation and myelosuppression, little is known about the mechanism by which the virus inhibits normal hematopoiesis, partly due to the lack of surrogate animal models. Current investigations suggest that CMV infection of CD34+ hematopoietic cells alters the bone marrow environment, promoting differentiation of myeloid cells and inhibiting lymphoid blood cell production [25]. Cytomegalovirus-induced production of inflammatory cytokines such as Il-10 seems to contribute to immunoparesis [26].
Antivirals available to control CMV can exacerbate myelosuppression, resulting in leukopenia and increased risk of fungal or bacterial infections. This unfortunate side effect seems to be relatively common and examples can be found in several published cases of Good’s syndrome with CMV reactivation, including a case of colitis in which foscarnet had to be stopped because of anemia and leukopenia, and a case of retinitis with ganciclovir-induced severe lymphopenia, also requiring discontinuation of the offending drug [27].
For this reason, it becomes difficult to make definite recommendations on CMV prophylaxis and preemptive treatment; current guidelines suggest that a history of tissue-invasive disease and serostatus should guide prophylaxis and, after receiving appropriate treatment, secondary prophylaxis with valganciclovir should be used. In cases where there is a perceived higher risk of drug-induced leukopenia, a preemptive approach constituted of serial (weekly or biweekly) monitoring of quantitative CMV DNA while using aciclovir for HSV and VZV prophylaxis may be employed [24]. Treatment with IVIG has also proven useful against CMV reactivation is some cases [28], and several articles suggest that the combination of IVIG and antiviral agents is the optimal form of secondary prophylaxis [22],[29],[30]. However, CMV-related aplasia in the context of Good’s syndrome remains an under-discussed topic and there are currently no official recommendations regarding its treatment or secondary prophylaxis.
CONCLUSION
The multifactorial nature of thymoma-related immunodeficiency merits a careful and tactical approach, knowing that lifting of immunosuppressive drugs may prompt autoimmune exacerbation. These patients require close monitoring and a long-term follow-up, as Good’s syndrome and other parathymic syndromes seldom resolve upon thymectomy, possibly recurring several years after thymoma removal, often presenting with an opportunistic infection. Cytomegalovirus reactivation in these patients is not infrequent, and its role in perpetuating immunosuppression and cytopenias may be underappreciated. Unfortunately, although there have been several reports of severe infections by CMV and other herpes viruses, diagnosis is often considered late in the course of the disease with limited treatment success, underlining the need to raise awareness to this condition, which may happen at any time in patients with a history of thymoma.
REFERENCE
1.
Zdrojewicz Z, Pachura E, Pachura P. The thymus: A forgotten, but very important organ. Adv Clin Exp Med 2016;25(2):369–75. [CrossRef]
[Pubmed]

2.
Shelly S, Agmon-Levin N, Altman A, Shoenfeld Y. Thymoma and autoimmunity. Cell Mol Immunol 2011;8(3):199–202. [CrossRef]
[Pubmed]

3.
Lippner EA, Lewis DB, Robinson WH, Katsumoto TR. Paraneoplastic and therapy-related immune complications in thymic malignancies. Curr Treat Options Oncol 2019;20(7):62. [CrossRef]
[Pubmed]

4.
Rosenow EC 3rd, Hurley BT. Disorders of the thymus. A review. Arch Intern Med 1984;144(4):763–70.
[Pubmed]

5.
Holbro A, Jauch A, Lardinois D, Tzankov A, Dirnhofer S, Hess C. High prevalence of infections and autoimmunity in patients with thymoma. Hum Immunol 2012;73(3):287–90. [CrossRef]
[Pubmed]

6.
Kelleher P, Misbah SA. What is Good’s syndrome? Immunological abnormalities in patients with thymoma. J Clin Pathol 2003;56(1):12–6. [CrossRef]
[Pubmed]

7.
Malphettes M, Gérard L, Galicier L, et al. Good syndrome: An adult-onset immunodeficiency remarkable for its high incidence of invasive infections and autoimmune complications. Clin Infect Dis 2015;61(2):e13–9. [CrossRef]
[Pubmed]

8.
Vitiello L, Masci AM, Montella L, et al. Thymoma-associated immunodeficiency: A syndrome characterized by severe alterations in NK, T and B-cells and progressive increase in naïve CD8+ T cells. Int J Immunopathol Pharmacol 2010;23(1):307–16. [CrossRef]
[Pubmed]

9.
Christopoulos P, Fisch P. Acquired T-cell immunodeficiency in thymoma patients. Crit Rev Immunol 2016;36(4):315–27.
[Pubmed]

10.
Tamburello A, Castelnovo L, Faggioli P, et al. Good’s syndrome, a rare form of acquired immunodeficiency associated with thymomas. Clin Pract 2019;9(2):1112. [CrossRef]
[Pubmed]

11.
Del Pino Molina L, Wentink M, van Deuren M, van Hagen PM, Smith CIE, van der Burg M. Precursor B-cell development in bone marrow of Good syndrome patients. Clin Immunol 2019;200:39–42. [CrossRef]
[Pubmed]

12.
Sierra H, Cordova M, Chen CSJ, Rajadhyaksha M. Confocal imaging-guided laser ablation of basal cell carcinomas: An ex vivo study. J Invest Dermatol 2015;135(2):612–5. [CrossRef]
[Pubmed]

13.
Zaman M, Huissoon A, Buckland M, et al. Clinical and laboratory features of seventy-eight UK patients with Good’s syndrome (thymoma and hypogammaglobulinaemia). Clin Exp Immunol 2019;195(1):132–8. [CrossRef]
[Pubmed]

14.
Dong JP, Gao W, Teng GG, Tian Y, Wang HH. Characteristics of Good’s syndrome in China: A systematic review. Chin Med J (Engl) 2017;130(13):1604–9. [CrossRef]
[Pubmed]

15.
Means Jr RT. Pure red cell aplasia. Hematology Am Soc Hematol Educ Program 2016;2016(1):51–6. [CrossRef]
[Pubmed]

16.
Wang S, Breskovska I, Gandhy S, Punga AR, Guptill JT, Kaminski HJ. Advances in autoimmune myasthenia gravis management. Expert Rev Neurother 2018;18(7):573–88. [CrossRef]
[Pubmed]

17.
Murrell GA, Rapeport WG. Clinical pharmacokinetics of allopurinol. Clin Pharmacokinet 1986;11(5):343–53. [CrossRef]
[Pubmed]

18.
Kamata T, Yoshida S, Wada H, et al. Extrathymic malignancies associated with thymoma: A forty-year experience at a single institution. Interact Cardiovasc Thorac Surg 2017;24(4):576–81. [CrossRef]
[Pubmed]

19.
Evoli A, Lancaster E. Paraneoplastic disorders in thymoma patients. J Thorac Oncol 2014;9(9 Suppl 2):S143–7. [CrossRef]
[Pubmed]

20.
Kahraman A, Miller M, Maldonado-Lopez E, Baba HA, Treichel U, Gerken G. A 55-year-old woman with thymoma and hypogammaglobulinemia (Good syndrome), ulcerative colitis, and cytomegalovirus infection. [Article in German]. Med Klin (Munich) 2009;104(2):150–4. [CrossRef]
[Pubmed]

21.
Huissoon AP, Davies G, Cox RA, Sloper CML, Thomson BJ, Robins RA. Loss of cytomegalovirus-specific immunological memory in a patient with thymoma. Clin Exp Immunol 2002;129(2):297–301. [CrossRef]
[Pubmed]

22.
Tarr PE, Sneller MC, Mechanic LJ, et al. Infections in patients with immunodeficiency with thymoma (Good syndrome). Report of 5 cases and review of the literature. Medicine (Baltimore) 2001;80(2):123–33. [CrossRef]
[Pubmed]

23.
Shiraishi J, Tsugata M, Masuda R, Mori Y, Suzuki K, Takemura T. Type AB thymoma accompanied by pure red cell aplasia and Good syndrome with CMV infection of tumor cells. Pathol Int 2008;58(8):489–93. [CrossRef]
[Pubmed]

24.
Multani A, Gomez CA, Montoya JG. Prevention of infectious diseases in patients with Good syndrome. Curr Opin Infect Dis 2018;31(4):267–77.
[Pubmed]

25.
Crawford LB, Tempel R, Streblow DN, et al. Human cytomegalovirus infection suppresses CD34+ progenitor cell engraftment in humanized mice. Microorganisms 2020;8(4):525. [CrossRef]
[Pubmed]

26.
Zischke J, Mamareli P, Pokoyski C, et al. The human cytomegalovirus glycoprotein pUL11 acts via CD45 to induce T cell IL-10 secretion. PLoS Pathog 2017;13(6):e1006454. [CrossRef]
[Pubmed]

27.
Mateo-Montoya A, Stanescu D, Wolff B, Sahel JA, Bonnel S. Cytomegalovirus retinitis associated with Good’s syndrome. Eur J Ophthalmol 2010;20(2):479–80. [CrossRef]
[Pubmed]

28.
Thongngarm T, Boonyasiri A, Pradubpongsa P, et al. Features and outcomes of immunoglobulin therapy in patients with good syndrome at Thailand’s largest tertiary referral hospital. Asian Pac J Allergy Immunol 2019;37(2):109–15. [CrossRef]
[Pubmed]

29.
Koriyama N, Fukumoto O, Fukudome M, et al. Successful treatment of Good syndrome with cytomegalovirus duodenoenteritis using a combination of ganciclovir and immunoglobulin with high anti-cytomegalovirus antibody titer. Am J Med Sci 2004;327(1):49–54.
[Pubmed]

30.
Ueno S, Sekimoto-Tsuboi S, Ishiguro Y, et al. Good’s syndrome with opportunistic infection of the central nervous system: A case report. BMC Neurol 2015;15:150. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Acknowledgement
We would like to thank the staff of Departments of Dermatology and Anatomic Pathology, specifically the Laboratory of Cutaneous Histopathology of the Santa Maria University Hospital, with particular thanks to Professor Soares de Almeida for providing the pathology images and their description.
Author ContributionsMaria Cunha - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Inês Leitão - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Tiago Marques - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Álvaro Pereira - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2021 Maria Cunha et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.