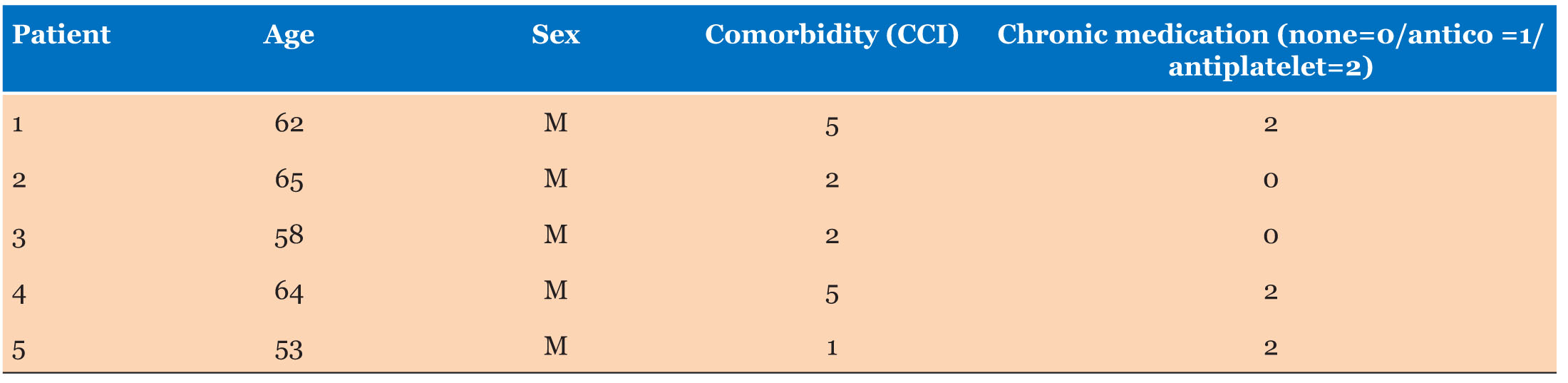
|
Case Report
Laminar heterotopia: A rare cause of epilepsy and mental retardation
1 Resident Physician, Radiology Department, UHC Ibn Sina, Rabat, Morocco
2 Professor at the Radiology, Mohamed V Military Instruction Hospital, Rabat, Morocco
Address correspondence to:
Merbouh Sahar
CHU Ibn Sina, Rabat,
Morocco
Message to Corresponding Author
Article ID: 100114Z06MS2023
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Sahar M, Dokal DI, Hassan N. Laminar heterotopia: A rare cause of epilepsy and mental retardation. Case Rep Int 2023;12(1):9–11.ABSTRACT
Neuronal migration disorders are a category of developmental brain disorders leading to cortical dysplasia. Laminar heterotopia is a form of diffuse gray matter heterotopia, which can result from a failure of proliferation, migration, or organization of neuronal and glial cells in the developing cortex leading to cortical dysplasia. The etiopathogenesis of this malformation remains a subject of discussion. Band heterotopia or double cortex are frequently shown if not always of genetic origin (mutation of genes coding for neuronal migration). We report the case of a young patient who presented episodes of complex seizures since childhood and mild mental retardation.
Keywords: Double cortex, Laminar heterotopia, Magnetic resonance imaging
INTRODUCTION
Subcortical laminar heterotopia also called as band heterotopia or double cortex consists of bilateral extensive plates of heterotopic gray matter located between the cortex and the cerebral ventricles [1]. It is an X-linked disorder (located on Xq22 or Xq21–24) with a dominant inheritance. Laminar heterotopia results from a cortical dysgenesis disorder associated with a defect in neuronal migration [2], affected individuals typically present with mental retardation and epilepsy. The brain malformation is often detected after onset of seizures in childhood. The overwhelming majority of affected patients are females, although rare males have been described.
CASE REPORT
We report the case of a 16-year-old male patient presented with sudden disturbance of consciousness, complex seizures since the age of ten, moderate mental and intellectual retardation, no consanguinity was noted, and there was no family history of epilepsy or other neurological disorders.
Physical and neurologic examination was normal. Interictal electroencephalograms (EEG) study revealed presence of frequent epileptiform discharges over bilateral parietotemporal regions.
Magnetic resonance imaging brain (Figure 1 and Figure 2) revealed a symmetric band of gray matter paralleling the cortex and subcortical white matter in both cerebral hemispheres. The abnormal band of gray matter displayed similar signal intensity to cortex on all pulse sequences suggestive of band heterotopia, giving a double cortex image. No other structural abnormality was seen on magnetic resonance imaging.
DISCUSSION
Subcortical band heterotopia was defined as a neurodevelopmental disorder that consists of bilateral and symmetric ribbons of gray matter located in the centrum semiovale between the cortex and ventricular walls, which are separated from both by layers of white matter [3].
Band heterotopia is a rare cortical malformation seen predominantly in females and considered as a mild form of lissencephaly [4]. Patients commonly present with epilepsy and possible delayed milestones and/or mental retardation [5].
The seizure types are highly variable from patient to patient and vary from focal, complex partial to generalized seizures [5]. Although patients with the more severe forms often present during infancy, patients may present at any age from newborn to adulthood. The relative thickness of the heterotopic band correlates with the phenotype and the severity of intellectual disability varies and depends on the thickness of the heterotopic band, patients with thicker bands have more severe mental retardation and seizures [1],[2],[3],[4],[5],[6].
Subcortical band heterotopia may be familial, with X-linked dominant inheritance and results from an early arrest of neuronal migration. There is no specific pattern of EEG findings described in the current literature [7].
On magnetic resonance imaging, it shows the characteristic 3-layer cake called “double cortex,” which is characterized by an extensive linear symmetric circumferential subcortical neuronal heterotopia parallel to the cortical surface. The cortex may be relatively normal or pachygyric [5]. The laminar heterotopic gray matter remains isointese to cortical surface on all sequences and do not enhance after administration of paramagnetic contrast [8].
Neuronal migration disorders, including laminar and nodular (subcortical or periventricular) heterotopia, have been recognized by neuropathologists for many years. The causes of neural migration disorders remain unknown. Vascular and toxic causes have been suspected [9].
Antiepileptic medication corresponding to the symptomatic generalized epilepsy with focalization is the mainstay of treatment for SBH. Antiepileptic drugs should be selected according to each patient’s seizure types. However callosotomy is not capable of eliminating seizures, because of its palliative nature; nevertheless, it would be worth considering for patients with frequent drop attacks [10].
CONCLUSION
Subcortical laminar heterotopia (SCLH), or “double cortex,” is a cortical dysgenesis disorder associated with a defect in neuronal migration. It is revealed by epilepsy associated or not with psychomotor retardation. Magnetic resonance imaging makes a positive diagnosis, assess the thickness of the heterotopic band, and looks for associated malformations.
REFERENCE
1.
Dobyns WB, Andermann E, Andermann F, et al. X-linked malformations of neuronal migration. Neurology 1996;47(2):331–9. [CrossRef]
[Pubmed]

2.
Keraliya A, Naphade P, Murphy D. Band heterotopia: An unusual cause of seizures. Ir Med J 2016;109(5):411.
[Pubmed]

3.
Abdel Razek AA, Kandell AY, Elsorogy LG, Elmongy A, Basett AA. Disorders of cortical formation: MR imaging features. AJNR Am J Neuroradiol 2009;30(1):4–11. [CrossRef]
[Pubmed]

4.
5.
Mahmud R. Subcortical band heterotopia presented with refractory epilepsy and reversible aphasia. Cureus 2021;13(8):e16990. [CrossRef]
[Pubmed]

6.
Barkovich AJ, Raybaud CA. Neuroimaging in disorders of cortical development. Neuroimaging Clin N Am 2004;14(2):231–54, viii. [CrossRef]
[Pubmed]

7.
Pinard JM, Motte J, Chiron C, Brian R, Andermann E, Dulac O. Subcortical laminar heterotopia and lissencephaly in two families: A single X linked dominant gene. J Neurol Neurosurg Psychiatry 1994;57(8):914–20. [CrossRef]
[Pubmed]

8.
Tanaka T, Gleeson JG. Subcortical laminar (band) heterotopia. Handb Clin Neurol 2008;87:191–204. [CrossRef]
[Pubmed]

9.
des Portes V, Francis F, Pinard JM, et al. Doublecortin is the major gene causing X-linked subcortical laminar heterotopia (SCLH). Hum Mol Genet 1998;7(7):1063–70. [CrossRef]
[Pubmed]

10.
des Portes V, Pinard JM, Smadja D, et al. Dominant X linked subcortical laminar heterotopia and lissencephaly syndrome (XSCLH/LIS): Evidence for the occurrence of mutation in males and mapping of a potential locus in Xq22. J Med Genet 1997;34(3):177–83. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Merbouh Sahar - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Diallo Ibrahima Dokal - Conception of the work, Design of the work, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Nouali Hassan - Conception of the work, Design of the work, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2023 Merbouh Sahar et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.