Case Report
Cavernous hemangioma of the inferior vena cava: A first case report
1 Surgery Resident, Hospital Ángeles del Pedregal, Mexico City, Mexico
2 Medical Degree, Hospital Ãngeles del Pedregal, Mexico City, Mexico
3 Surgery Resident, Hospital Ángeles del Pedregal, Mexico City, Mexico
4 Surgery Resident, Hospital Ángeles del Pedregal, Mexico City, Mexico
5 Oncologist Surgeon, Hospital Ãngeles del Pedregal, Mexico City, Mexico
6 Oncologist Surgeon, Hospital Ãngeles del Pedregal, Mexico City, Mexico
Address correspondence to:
Francisco José Flores-Palomar
Chabacano 20A #4, Magdalena Contreras, 10810, Mexico City,
Mexico
Access full text article on other devices

Access PDF of article on other devices

Article ID: 100065Z06FP2019
doi: 10.5348/100065Z06FP2019CR
How to cite this article
Flores-Palomar FJ, Raphael-Garza MJ, De-la-Concha-Tiznado M, Lara-Ruiz I, de Oca-Orellana CRM, Alfeiran-Ruiz A. Cavernous hemangioma of the inferior vena cava: A first case report. Case Rep Int 2019;8:100065Z06FP2019.ABSTRACT
Introduction: Vascular malformations are anomalies of the vascular system, constituted by a network of vessels with mature endothelium, with normal cell replacement without proliferation capacity.
Case Report: We report the clinical case of a 25-year-old man who came to the hospital for left pelvic limb edema and lumbar pain. An ultrasonography (USG) and positron emission tomography-computed tomography (PET-CT) revealed deep vein thrombosis of the inferior vena cava (IVC) and left iliac vein and a 9.5 cm × 5 cm retroperitoneal nometabolic tumor compatible with perivascular lymphadenopathies. Exploratory laparotomy was performed, transperitoneum exploration of the retroperitoneum and resection of the tumor arising from the IVC and left iliac vein. Pathological examination revealed a 10 cm retroperitoneal tumor filled with venous vessels and fibrotic septa. Postoperative diagnosis was venous malformation of the IVC.
Conclusion: Cavernous hemangiomas are not true vascular tumors, but rather a congenital vascular anomaly. It is classified by International Society for the Study of Vascular Anomalies as venous malformation. This tumor is present from birth, and it grows with the child, they do not involute spontaneously. A differential diagnosis of vena cava tumors is leiomyosarcoma. This is the first case in literature of a vascular malformation (formerly called cavernous hemangioma) involving the IVC. It is important to know that this benign pathology has an excellent prognosis and survival after a successful surgery.
INTRODUCTION
Vascular malformations are anomalies of the vascular system, constituted by a network of vessels with mature endothelium, with normal cell replacement without proliferation capacity. They are present from birth and grow together with the patient, unlike the hemangiomas they do not involute spontaneously; these venous malformations (VM) are still wrongly named cavernous hemangiomas [1].
Although cavernous hemangiomas are most commonly located in the skin of the head or neck, theoretically they may be found in nearly all organs with vascular endothelial cells. Of the cases in non-skin tissues, the most common gastrointestinal hemangiomas are in the liver [2], other locations that have been described are in the heart [3], ileum [4], adrenal gland [5], mediastinum [6], in a persistent left superior vena cava [7] and the thymus [8].
A vascular malformation (cavernous hemangioma) of the IVC is an entity that, to our knowledge, has not been described in the literature before.
CASE REPORT
We are reporting a clinical case of a 25-year-old male. He came to the hospital with a history of three weeks prior admission with intermittent lumbar pain, and a 24 hour left limb increased in volume and pain (Figure 1). We performed blood test, with increased D-dimer with normal tumor markers (alpha-fetoprotein [AFP], carcinoembryonic antigen [CEA], and human chorionic gonadotropin [hCG]). On clinical examination the patient had significant increase in left limb volume, without other signs. So we decided to perform Doppler ultrasound that revealed and organized thrombus of the IVC, and left common iliac vein. We decided to rule out a paraneoplastic syndrome, so we performed a PET-CT that revealed a 9.5 cm by 5 cm no-metabolic mass, compatible with perivascular lymphadenopathies, associated with compressional phenomenon (Figure 2). Prior surgery it was decided to place a Greenfield filter in the IVC in the adrenal position. Magnetic resonance imagining (MRI) was not performed in this patient. The patient did not have clinical features suggestive malignancy.
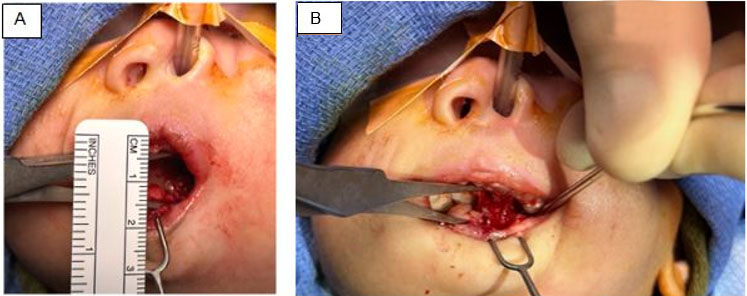
Exploratory laparotomy was performed a transperitoneal approach to the retroperitoneum, left medial visceral rotation of the sigmoid was made, to expose the mass and veins, the lesion was identified arising from the IVC (Figure 3A). It was entirely resected, and the veins were repaired with vascular suture, no implant needed (Figure 3B, C). Operative time was 70 minutes, with blood loss of 20 mL. The postoperative hospital stay was three days. Pathological examination revealed a 5 cm × 2.8 cm × 1.8 cm, weighted 16 g, macroscopically it was round, smooth, and red, with multiple dilated venous vessels with an average size of 0.5 cm, with fibrous tissue. Microscopically, venous vessels with fibrotic septa were found in the elastic fibers staining (Figure 4). There was no evidence of malignancy.



DISCUSSION
Cavernous hemangiomas are not true vascular tumors, but rather a congenital vascular anomaly. It is classified by International Society for the Study of Vascular Anomalies as VM in the slow-flow lesion category. It has been reported to arise at all sites including skin and subcutaneous layers of the head, neck, face, extremities, liver, and gastrointestinal tract [8]. This tumor is present from birth, and it grows with the child, they do not involute spontaneously. Regarding the predominant type of vessel, VM is differentiated into venules, veins, lymphatics, arteriovenous, and mixed, with high flow pattern (arteriovenous or mixed malformations) or low (capillary, venous, lymphatic, or mixed) [1].
Venous malformations are caused by mutations in the endothelial receptor tyrosine kinase TIE2. Mutations are located in the intracellular tyrosine kinase, kinase insert, or carboxyl-terminal tail, and result in amino acid substitutions or create C-terminal premature stop codons. They induce TIE2 receptor phosphorylation in the absence of a ligand [9].
A differential diagnosis of vena cava tumors is leiomyosarcoma, which is a rare tumor with 300 cases reported; it can cause venous compression and theore lower limb edema [10]. Retroperitoneal tumors of vascular origins are rare, the most frequent malignant tumors are liposarcomas, leiomyosarcomas, and benign are lipomas, leiomyoma, and cavernous hemangioma. For hepatic hemangioma embolization it is an option. But in retroperitoneum resection is the treatment of choice [4]. Another differential diagnosis is a tumor that invades the IVC, for example, renal angiomyolipoma, generally benign, also pheochromocytoma, renal cell carcinoma, adrenal carcinoma, hepatocellular carcinoma, and a pseudolipoma [11].
The diagnosis is frequently postoperative, with the presence of full vessels (blood filled), and dilated vascular spaces [5].
Lateral resection should not be performed in patients with primary caval tumors with extraluminal extension. With renal cell carcinoma with extensive intraluminal involvement, open thrombectomy alone, or patch resection of the IVC around the origin of the renal vein, carries the risk of late recurrence from venous wall [12].
This is the first case in literature of a vascular malformation (formerly called cavernous hemangioma) involving the IVC. This case was observed in a young patient, with signs of deep vascular thrombosis of the left pelvic limb. There is still confusion in naming this vascular tumor.
True hemangiomas grow by endothelial cell hyperplasia and should be differentiated from vascular malformations, which are not true neoplasms but are localized defects of vascular morphogenesis caused by dysfunction in embryogenesis and vasculogenesis [13].
It is important to know that this benign pathology has an excellent prognosis and survival after a successful surgery.
CONCLUSION
The vascular malformations are an entity that can arise from any part of the body. The correct study of a mass that is dependent or is near a great vessel, need to have in mind this pathology for a differential diagnosis, with probably vascular compression symptoms without malignancy signs.
REFERENCES
1.
Arce VJD, García BC, Otero OJ, Villanueva AE. Anomalías vasculares de partes blandas: Imágenes diagnósticas. Rev Chil Radiol 2007;13(3):109–21.

2.
Akbulut S, Yilmaz M, Kahraman A, Yilmaz S. Bilateral lower limb edema caused by compression of the retrohepatic inferior vena cava by a giant hepatic hemangioma. Int Surg 2013;98(3):229–33. [CrossRef]
[Pubmed]

3.
Fan C, Tan C, Kong D, Yang J, Yuan S, Wu S. A giant cavernous hemangioma of the left atrioventricular groove. Case Rep Pediatr 2017;2017:6898629. [CrossRef]
[Pubmed]

4.
Zielinski J, Haponiuk I, Jaworski R, Peksa R, Irga-Jaworska N, Jaskiewicz J. Retroperitoneal tumor: Giant cavernous hemangioma – case presentation and literature review. Kardiochir Torakochirurgia Pol 2016;13(4):375–9. [CrossRef]
[Pubmed]

5.
Agrusa A, Romano G, Salamone G, et al. Large cavernous hemangioma of the adrenal gland: Laparoscopic treatment. Report of a case. Int J Surg Case Rep 2015;16:150–3. [CrossRef]
[Pubmed]

6.
Kaya SO, Samancılar O, Usluer O, Acar T, Yener AG. Giant cavernous haemangioma of the anterior mediastinum. Eurasian J Med 2015;47(3):216–7. [CrossRef]
[Pubmed]

7.
Hu W, Wang X, Tan S, et al. Large hemangioma in a persistent left superior vena cava. Thorac Cardiovasc Surg 2012;60 Suppl 2:e3–5. [CrossRef]
[Pubmed]

8.
Kim JH, Choi JG, Son BC. Venous malformation (Cavernous Hemangioma) of the supraorbital nerve. Asian J Neurosurg 2018;13(2):499–502. [CrossRef]
[Pubmed]

9.
Queisser A, Boon LM, Vikkula M. Etiology and genetics of congenital vascular lesions. Otolaryngol Clin North Am 2018;51(1):41–53. [CrossRef]
[Pubmed]

10.
Alkhalili E, Greenbaum A, Langsfeld M, et al. Leiomyosarcoma of the inferior vena cava: A case series and review of the literature. Ann Vasc Surg 2016;33:245–51. [CrossRef]
[Pubmed]

11.
Molina M, Schiappacasse G, Labra A. Tumors that invade the inferior vena cava: An illustrative review of the main imaging features on computed tomography and magnetic resonance. Rev ChilRadiol 2016;22(1):39–46.

12.
Hardwigsen J, Baqué P, Crespy B, Moutardier V, Delpero JR, Le Treut YP. Resection of the inferior vena cava for neoplasms with or without prosthetic replacement: A 14-patient series. Ann Surg 2001;233(2):242–9. [CrossRef]
[Pubmed]

13.
George A, Mani V, Noufal A. Update on the classification of hemangioma. J Oral Maxillofac Pathol 2014;18(Suppl 1):S117–20. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Acknowledgement
We thank Magdalena Sofia Mercado-Fernandez for her help in translation.
Author ContributionsFrancisco José Flores-Palomar - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
María José Raphael-Garza - Substantial contributions to conception and design, Acquisition of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Mauricio De-la-Concha-Tiznado - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Drafting the article, Final approval of the version to be published
Iván Lara-Ruiz - Substantial contributions to conception and design, Revising it critically for important intellectual content, Final approval of the version to be published
Claudio Rene Monte de Oca-Orellana - Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Antonio Alfeiran-Ruiz - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Final approval of the version to be published
Data Availability StatementThe corresponding author is the guarantor of submission.
Consent For PublicationWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Competing InterestsAuthors declare no conflict of interest.
Copyright© 2025 Francisco José Flores-Palomar et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.